The study scheme is shown in Figure 1 (Clinical trial NCT03592472). The primary endpoint is progression-free survival. The secondary endpoints include progression-free survival by investigator assessment, overall survival, objective response rate, duration of response, response rate and duration of response in cross over patient’s population, patients reported outcomes/quality of life, and safety.
Figure 1 – Trial scheme:
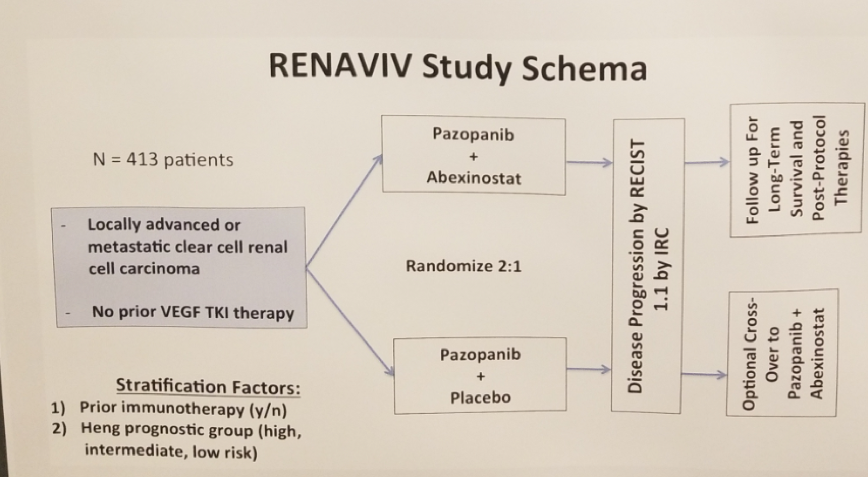
There are also some planned correlative studies, involving peripheral blood mononuclear cells, and archived tumor tissue. The first patient enrolled was in October 2018.
Presented by: Rahul Aggarwal, MD, University of California, San Francisco, California
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan, at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Aggarwal et al. Inhibiting Histone Deacetylase as a Means to Reverse Resistance to Angiogenesis Inhibitors: Phase I Study of Abexinostat Plus Pazopanib in Advanced Solid Tumor Malignancies. Journal of Clinical Oncology 35, no. 11 (April 10 2017) 1231-1239.


