Patients in this phase III study were randomized 1:1 to receive avelumab (10 mg/kg) IV every 2 weeks + axitinib (5 mg) PO twice daily (n=442) or sunitinib (50 mg) PO once daily for 4 weeks (6-wk cycle) (n=444). The trial design is as follows:

The primary and key secondary endpoints were PFS per independent review committee (IRC; RECIST v1.1) and OS in patients with PD-L1+ tumors (≥1% of immune cells) and in patients irrespective of PD-L1 expression. Additional secondary endpoints included objective response per IRC (RECIST v1.1). All subgroup analyses were prespecified in the analysis plan, except for body mass index, and smoking status, which were post-hoc exploratory analyses.
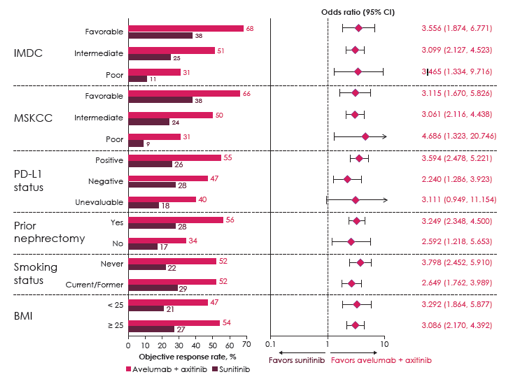
JAVELIN Renal 101 had a total of 886 patients were randomized, this included 560 patients (63%) that had PD-L1+ tumors (avelumab + axitinib, n=270; sunitinib, n=290). In the overall population, there were 64%/12% IMDC intermediate/poor risk patients treated with avelumab + axitinib compared to 66%/10% treated with sunitinib. The median follow-up for this analysis was 12.0 vs 11.5 months for avelumab + axitinib vs sunitinib groups. Stratified by IMDC prognostic group, avelumab + axitinib outperformed sunitinib in all risk groups: favorable (HR 0.54, 95%CI 0.321-0.907), intermediate (HR 0.74, 95%CI 0.570-0.950), and poor (HR 0.57, 95%CI 0.375-0.880). Similar results for prognostic risk were seen in patients with PD-L1+ tumors. 22.6% of patients on avelumab + axitinib had ≥1 type of follow-up anticancer therapy compared to 40.5% for patients on sunitinib; patients on avelumab + axitinib also outperformed sunitinib with PFS2 as an endpoint (HR 0.56, 95%CI 0.421-0.735). For all subgroups assessed, ORR favored avelumab + axitinib:
Dr. Choueiri concluded with several key take-home messages from JAVELIN Renal 101:
- This study demonstrated longer PFS and higher ORR with avelumab plus axitinib compared to sunitinib for treatment-naïve patients with advanced RCC
- PFS and ORR benefit was observed in patients regardless of PD-L1 status and in all prognostic groups
- Patients who received avelumab plus axitinib had longer PFS2 than those who received sunitinib
- The study continues to follow-up for OS
- The results support avelumab plus axitinib as a new first-line standard of care for patients with advanced RCC
Presented by: Toni K. Choueiri, Dana Farber Cancer Institute, Boston, Massachusetts
Co-Authors: Robert J. Motzer, Matthew T. Campbell, Boris Y. Alekseev, Motohide Uemura, Christian K. Kollmannsberger, Gwenaelle Gravis, Georg A. Bjarnason, Howard Gurney, Jinsoo Chung, John B. A. G. Haanen, Brian I. Rini, James M. G. Larkin, Manuela Schmidinger, Franco Nole, Aleksander Chudnovsky, Bo Huang, Subramanian Hariharan, Alessandra di Pietro, Laurence Albiges; Memorial Sloan Kettering Cancer Center, New York, NY; The University of Texas MD Anderson Cancer Center, Houston, TX; Moscow Scientific Research Oncology Institute, Moscow, Russian Federation; Osaka University Hospital, Osaka, Japan; British Columbia Cancer Agency, Vancouver, BC, Canada; Institut Paoli-Calmettes, Marseille, France; Odette Cancer Centre Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Macquarie University, Sydney, NSW, Australia; National Cancer Center, Goyang-Si, Korea, Republic of (South); Netherlands Cancer Institute, Amsterdam, Netherlands; Cleveland Clinic, Cleveland, OH; Royal Marsden NHS Foundation Trust, London, United Kingdom; Medical University Vienna, Department of Medicine I, Clinical Division of Oncology and Comprehensive Cancer Center, Vienna, Austria; Istituto Europeo Di Oncologia Medical Oncology Division of Urogenital and Head & Neck Tumours, Milano, Italy; Pfizer Inc., Cambridge, MA; Pfizer Inc., Groton, CT; Pfizer Inc., New York, NY; Pfizer SRL, Lombardia, Italy; Institut Gustave Roussy, Villejuif, France
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA


