This is retrospective analysis of 70 patients who were treated with a TKI after progression on first line ICI. Of note, all patients included in this study had been treated with front line ICI on a clinical trial at either MD Anderson and MSKCC.
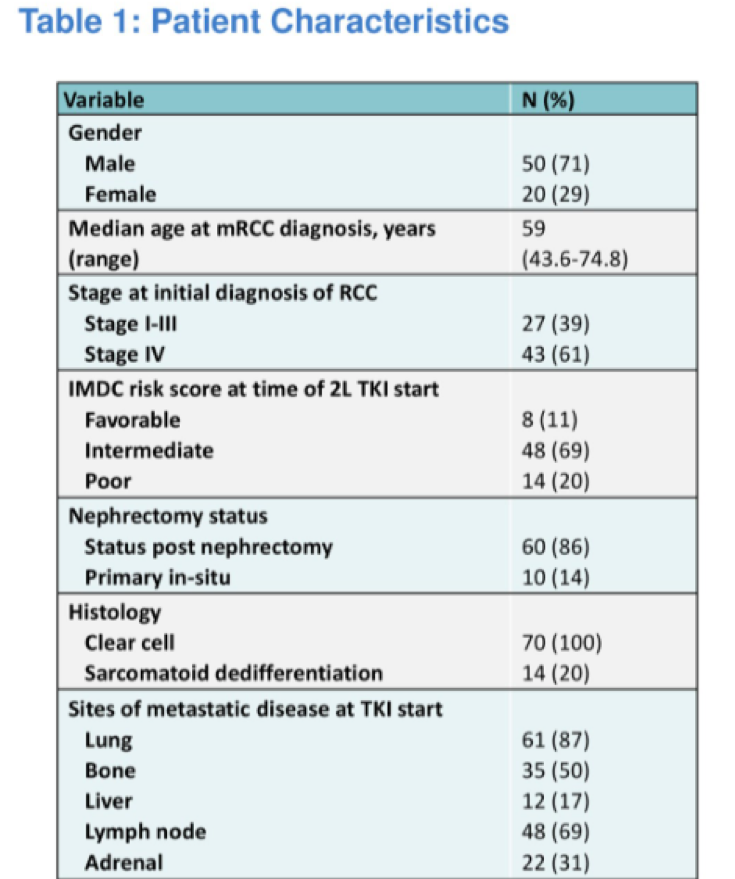
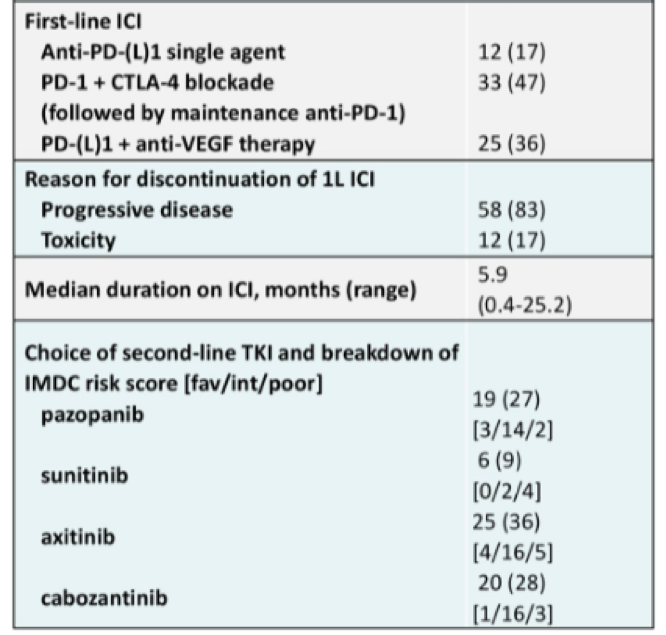
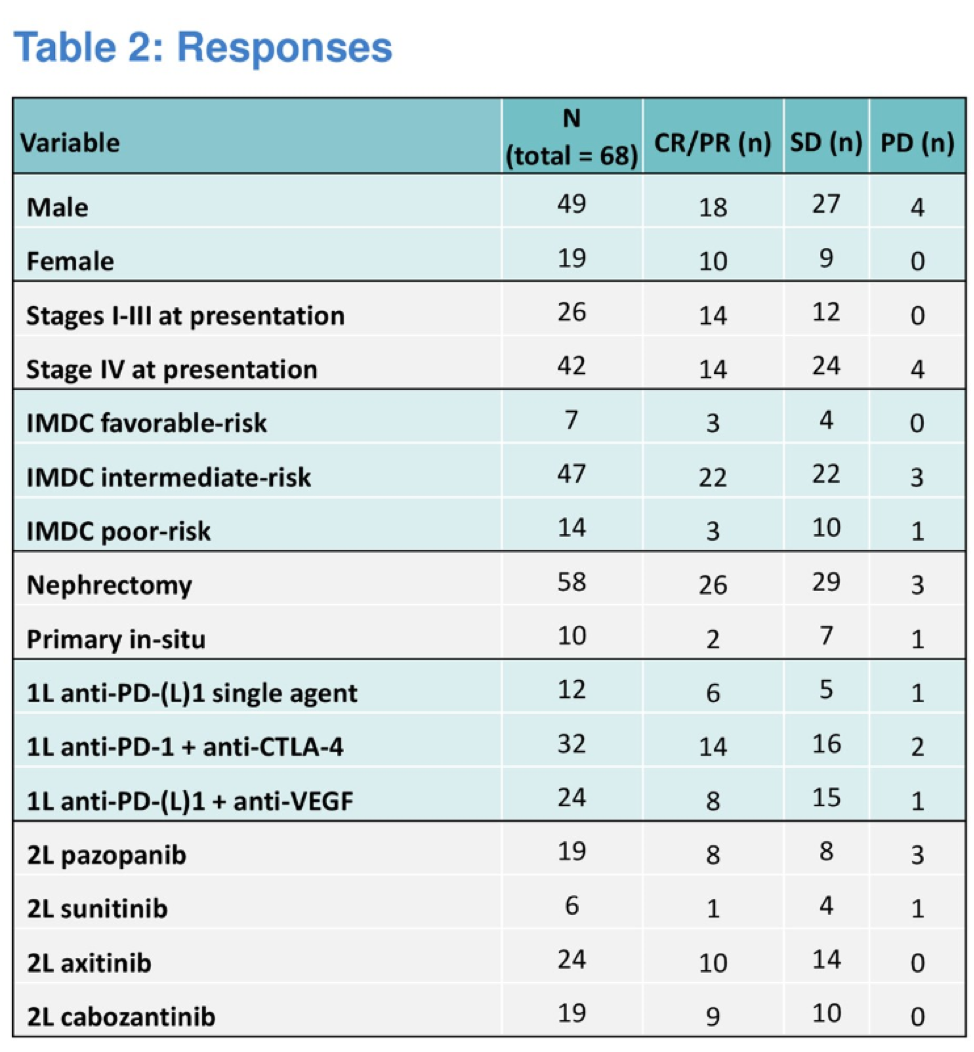
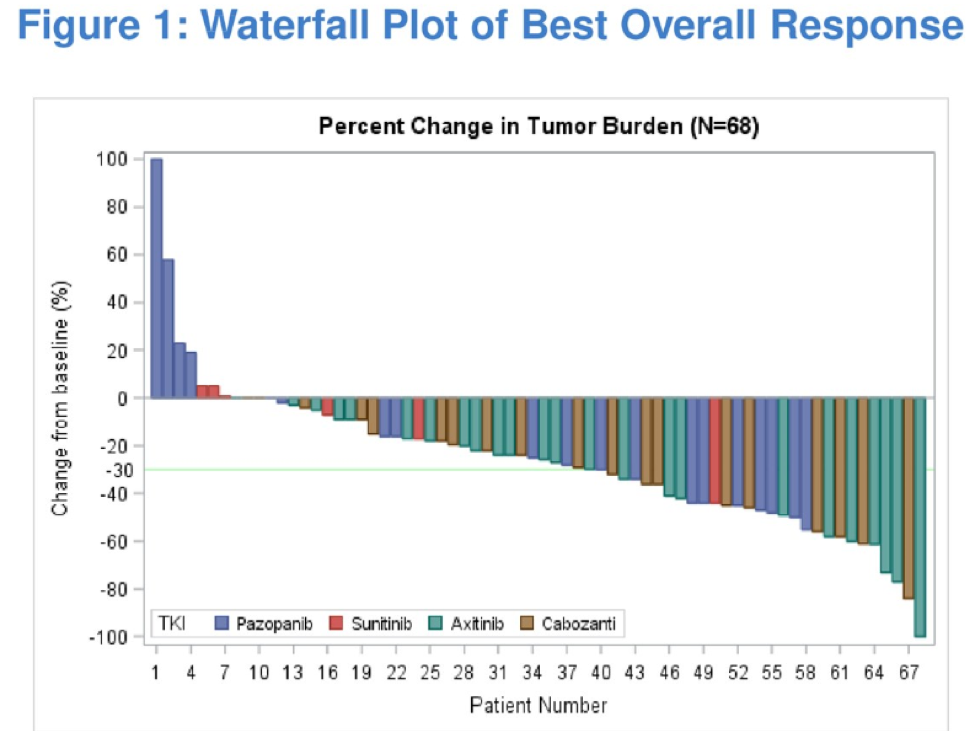
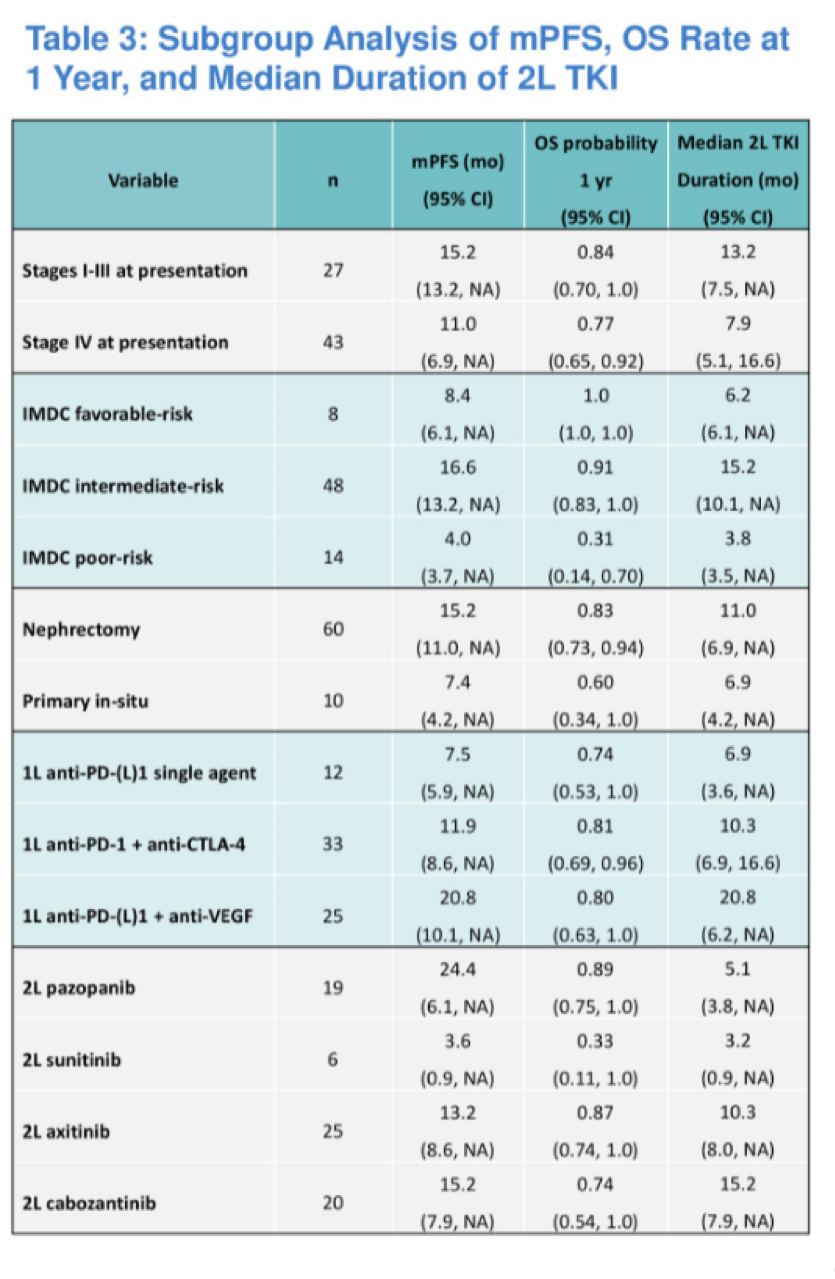
Median progression free survival was greatest for those treated with pazopanib (24.4 months), followed by 15.2 months for cabozantinib, 13.2 months for axitinib, and 3.6 months for sunitinib.
Presented by: Amishi Yogesh Shah, MD, Assistant Professor, MD Anderson Cancer Center
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:


