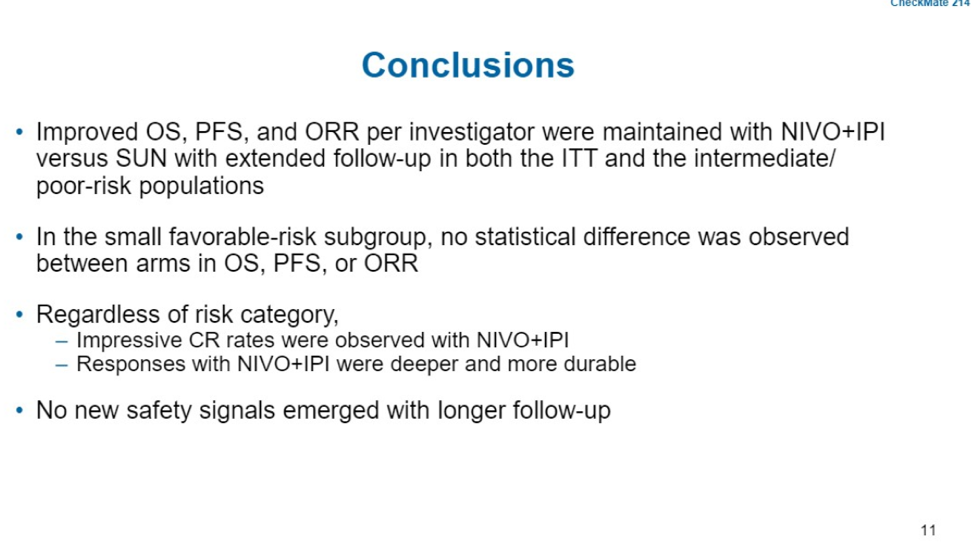
In CheckMate 214, patients received Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg every three weeks for 4 cycles, followed by nivolumab 3 mg/kg every 2 weeks. Patients randomized to sunitinib received sunitinib 50 mg on a 4 week on, 2 week off regimen. At this 30 month update, the overall survival benefit of ipi/nivo remains over sunitinib for the intention to treat cohort as well as for patients with intermediate or high risk mRCC. Median survival has not been reached, compared with 37.9 months for patients on sunitinib. At 30 months, overall survival was 64% in the ipi/nivo arm and 56% in the sunitinib arm.

For patients with favorable risk disease, this updated data shows that there was no significant difference in median OS, both groups not yet reached, HR 1.22, p=0.4426. For patients who had achieved a CR, 88% of patients in the intention to treat analysis continue to have a response. Even in patients with favorable risk, the CR was very durable and maintained in 9 of 10 patients.

In terms of safety, 35% of patients on ipi/nivo required high dose steroids for immune related treatment adverse events.

Since the publication of CheckMate 214, the combination of ipi/nivo has been rapidly adopted for intermediate and poor risk patients with mRCC. The data presented today also provides some evidence for utilizing ipi/nivo in good risk patients as well, because of increased number of complete responses compared with sunitinib, and the durability of those complete responses. At the end of the talk, based on this data, Dr. Tannir recommended that ipi/nivo may be used for all patients with mRCC, regardless of risk classification.
Presented by: Nizar M. Tannir, MD, FACP, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, MD Anderson Cancer Center
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:


