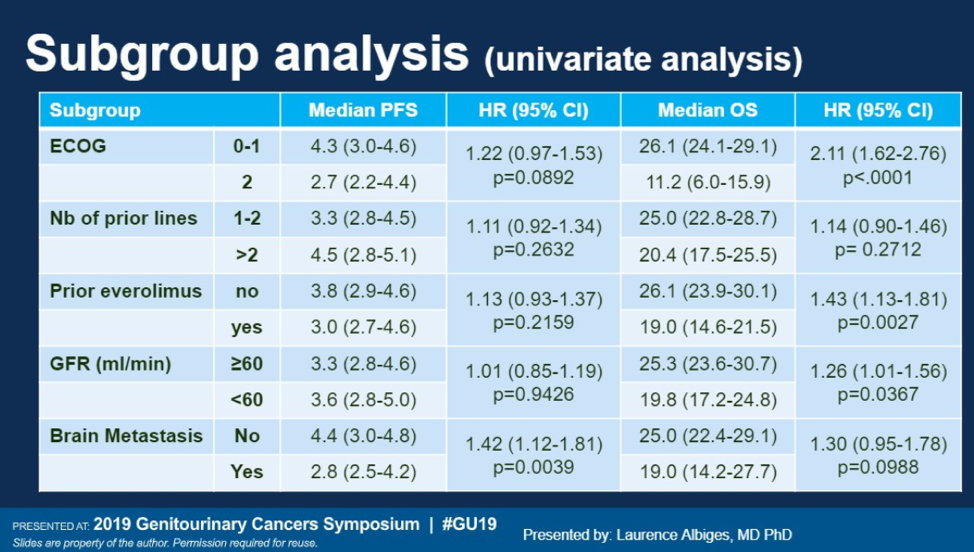
720 patients were included in the final analysis. In terms of patient characteristics, all patients had clear cell mRCC. The majority of patients were men (77.4%), had prior nephrectomy (84.7%), and had an ECOG of 0 (85%). 22.4% of patients had already received two prior lines of therapy, and the majority of patients were intermediate and poor risk (81.7%). 12.3% of patients were found to have brain metastases per study screening protocol.






Presented by: Laurence Albiges, MD, PhD, Head, Genitourinary Unit, Gustave Roussy Institute, Villejuif, France
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378:1277-90.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2015;373:1803-13


