TAS-115, a novel multi-kinase (e.g., MET, VEGFR) inhibitor has also shown to inhibit Feline McDonough Sarcoma oncogene (FMS) kinase, essential for the differentiation of osteoclasts, and is expected to be effective against bone metastases. Anti-tumor activity against bone metastases was observed in the preclinical and phase1 study. In this phase 2 study, the efficacy and safety of TAS-115 were evaluated in castration-resistant prostate cancer (CRPC) patients with bone metastases.
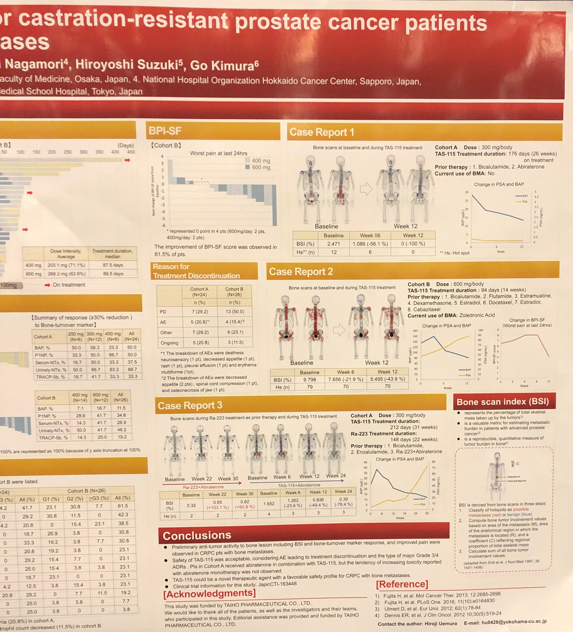
This was a phase 2, open-label, multi-arm study for CRPC patients (pts) with bone metastases to evaluate the clinical anti-tumor activity of TAS-115. Patients who had visceral metastases were ineligible for this study. TAS-115 was given orally, once daily, and schedule of 5 days on/2 days off each week was repeated. This study had two cohorts (A and B). In cohort A, TAS-115 ranging from 200 to 400 mg/day with abiraterone acetate was given to pts before docetaxel. In cohort B, CRPC pts who had symptomatic bone metastases, post or unfit to docetaxel, were randomized in a 1:1 to 400 or 600 mg/day of TAS-115. The primary endpoint was a bone scan index (BSI) response rate at week 12, defined as ≥ a 30% decrease of BSI from baseline. BSI is a quantitative assessment of bone scan data calculated by software (BONE NAVI).
From Nov 2016 to Jul 2018, a total of 50 pts received TAS-115 (24 pts in cohort A and 26 pts in cohort B). BSI response rate at week 12 and best BSI response was 20.8 % and 37.5% in cohort A, and 15.4 % and 19.2% in cohort B, respectively. Any reduction of BSI was observed in 70.8% of cohort A and 61.5% of cohort B. In cohort B; the pain was assessed using the Brief Pain Inventory-short form and improvement was observed in 61.5% of pts. Furthermore, the reduction of bone-turnover marker (BAP, P1NP, NTx, and TRACP-5b) was observed in both cohorts, but not PSA response. The major (≥ 10%) Grade 3/4 treatment-related adverse drug reactions were hypophosphatemia (21%) in cohort A, and anemia (23%), hypophosphatemia (12%) and neutrophil count decreased (12%) in cohort B.
Dr. Uemura concluded by noting that preliminary anti-tumor activity to the bone lesion and improved pain were observed in CRPC pts with bone metastases, and safety of TAS-115 was acceptable. TAS-115 could be a novel therapeutic agent with a favorable safety profile for CRPC with bone metastases. The poster is shown in the pictures below which also included three impressive case reports showing bone scans at baseline and during TAS-115 treatment
Dr. Uemura's Poster:
 .
.
Presented by: Hiroji Uemura, Professor, Department of Urology and Renal Transplantation, Yokohama City University Medical, Yokohama, Japan
Written by: Abhishek Srivastava, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Philadelphia, PA at the 2018 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, February 8-10, 2018 - San Francisco, CA


