
This abstract provides the initial results from Checkmate 650, a phase II study of ipilimumab/nivolumab for asymptomatic or minimally symptomatic patients with mCRPC, both before (cohort 1) and after chemotherapy (cohort 2). Patients were first treated with the combination of both checkpoint inhibitors (nivolumab 1 mg/kg + ipilimumab 3 mg/kg) every three weeks for four doses, followed by nivolumab 480 mg every 4 weeks. The authors created a coprimary endpoint of objective response rate (ORR) and radiographic PFS per PCWG2 and safety was a secondary endpoint. Results from the first 90 patients are reported here.

Baseline characteristics are shown above. The median age was 69 in cohort 1 and 65 in cohort 2. Cohort 1 had more patients without bone metastases (20% vs 6.7%) and cohort 2 had greater chemotherapy exposure, with 82% of patients having received docetaxel.
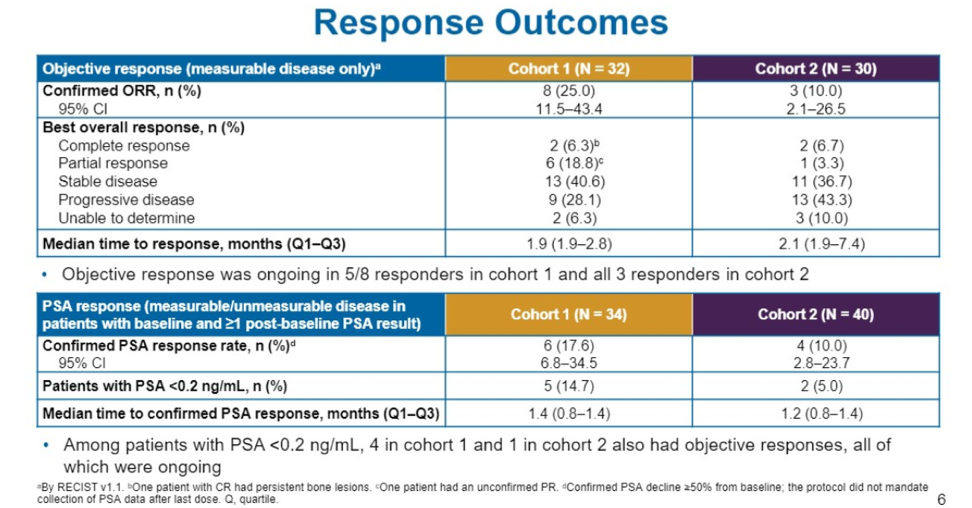
In terms of response outcomes, the confirmed ORR was 25% in cohort 1 and 10% in cohort 2. 2 (6%) patients in each cohort achieved a complete response. Patients who responded typically achieved a response very quickly, in about 2 months in both cohorts.
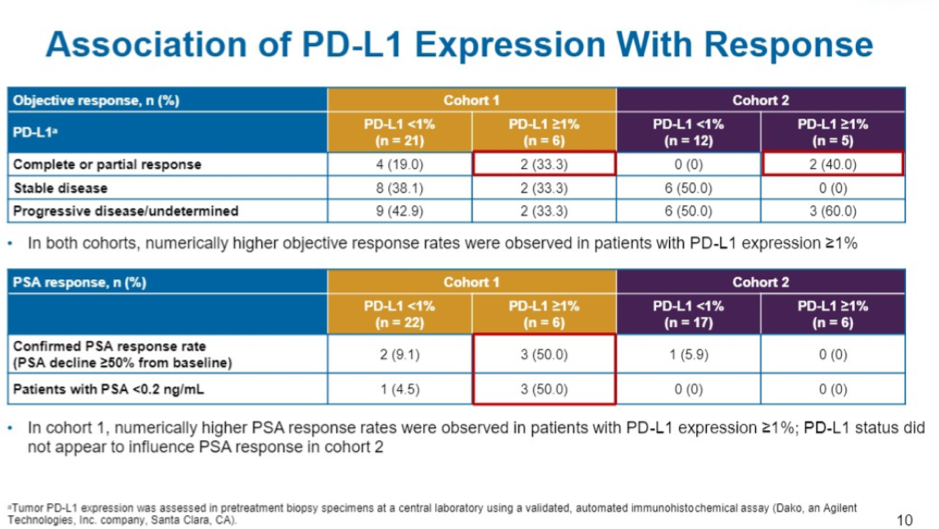
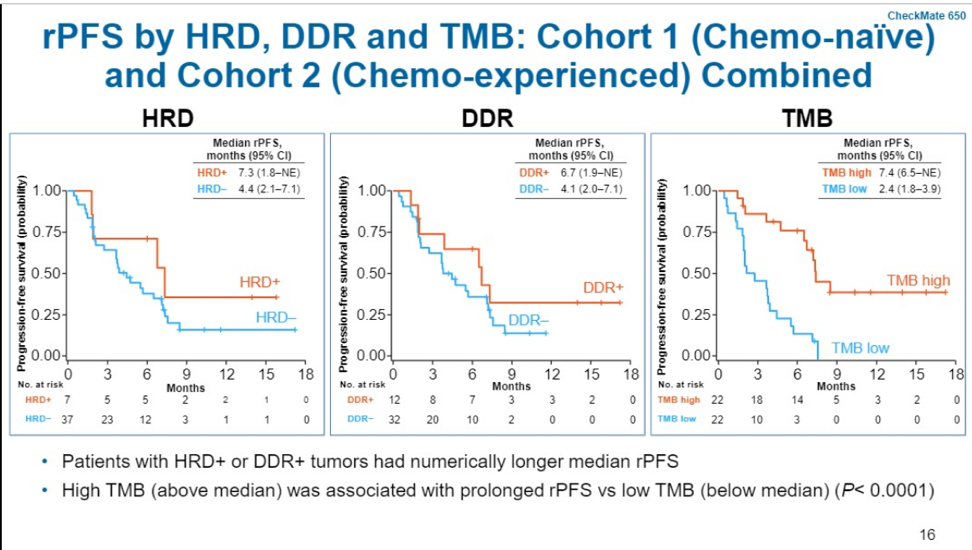
In terms of exploratory biomarker analysis, tumor PD-L1 ≥1% appeared to enrich for responders – 4/12 patients (33%). Tumors with mutations in DNA damage repair genes also were enriched for responders (40%, 4/10). TMB status was quantified based on whether or not the tumor was above or below the median TMB in the cohort which was 74.5 mutations/pt. Of those classified as TMB high, ORR was 56% (9/16).
In terms of safety, 40-50% of all patients experienced grade 3-5 toxicity, most common being diarrhea, fatigue, and rash, as has been seen in other tumor types. However, it is notable that 40-50% of all patients discontinued study drug due to toxicity and the median duration of therapy was 2.1 months in cohort 1 and 1.4 months in cohort 2. 
Ipilimumab/nivolumab is an active therapy for a small proportion of patients with mCRPC, both before and after chemotherapy. TMB high, PD-L1≥1%, DDR, and HRD mutations may enrich for responders and future biomarker work is critical to identify the population of patients who will best respond to checkpoint inhibition. A significant proportion of patients discontinued treatment due to toxicity. It is unknown if a different dosing regimen (ipi1/nivo3) or dosing schedule may be more tolerable. Long term follow-up is important to see if this combination may provide a durable response, as we have seen in melanoma and renal cell carcinoma.
Presented by: Padmanee Sharma, MD, PhD, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, MD Anderson Cancer Center
Discussant: William Kevin Kelly, DO
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine 2017;377:1345-56.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. New England Journal of Medicine 2015;372:2018-28.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine 2018;378:1277-90.
- Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New England Journal of Medicine 2017;376:1015-26.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine 2012;366:2443-54.
- Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016;7:52810-7.
- De Bono JS, Goh JC, Ojamaa K, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). American Society of Clinical Oncology; 2018.


