Dr. Shilpa Gupta presented a multi-center phase II study to evaluate the efficacy and safety of utilizing nivolumab with gemcitabine-cisplatin as neoadjuvant therapy. Nivolumab is a human IgG4 monoclonal antibody that blocks PD-1. It has been approved in patients with metastatic urothelial cancer progressing after platinum-based chemotherapy. In the study, 41 patients with muscle-invasive bladder cancer (cT2-T4a, N≤1, M0) who were candidates for radical cystectomy were enrolled. Patients received cisplatin on day 1; gemcitabine on days 1 and 8; and nivolumab on day 8 every 21 days for 4 cycles followed by radical cystectomy within 8 weeks. The primary objective was assessment of pathologic response (≤pT1N0). The secondary objective was assessment of safety.
The study design is depicted:
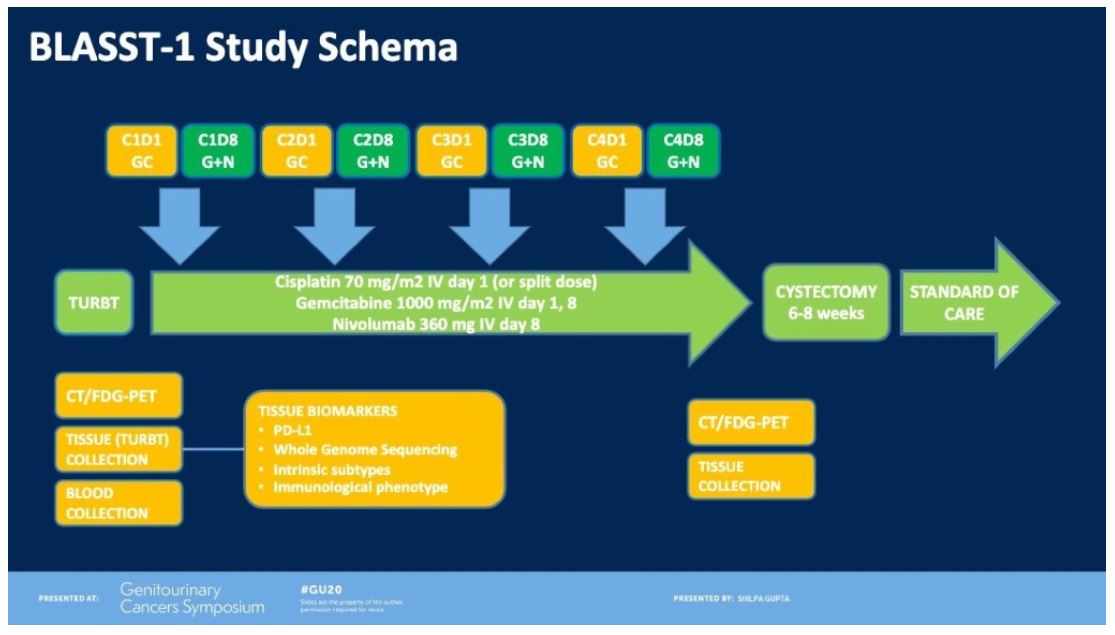
Of 41 patients (cT2N0 90%, cT3N0 7%, and cT4N1 3%), pathologic response occurred in 27/41 (66%) patients. Pathologic complete response (pT0, pTis) occurred in 20/41 (49%). With regards to safety, the rate of grade 3-4 adverse events was 10/41 (24%) with the majority of them attributed to gemcitabine and cisplatin (neutropenia, thrombocytopenia, and renal insufficiency). There were 5/41 (12%) patients with any grade adverse events attributed to immunotherapy. One patient developed a rash, 1 patient developed hypothyroidism, and 2 patients developed inflamed lymph nodes (all grade 1 adverse events). One patient developed Guillain-Barre Syndrome, which was successfully treated with intravenous immunoglobulin. None of the patients undergoing nivolumab in addition to standard neoadjuvant chemotherapy experienced a delay in time to radical cystectomy and there were no unexpected surgical complications attributable to this neoadjuvant treatment regimen.
Dr. Gupta concluded that neoadjuvant nivolumab in combination with gemcitabine-cisplatin is associated with significant pathologic downstaging rates. Also, utilization of this neoadjuvant treatment regimen is safe and associated with minimal added morbidity. Currently, a randomized phase III trial (ENERGIZE) is underway to confirm the results of this study.
Presented by: Shilpa Gupta, MD, Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland, Ohio
Written By: Ziho Lee, MD, Fellow in Advanced Robotic Oncology and Reconstruction, Temple University, Philadelphia, PA, Twitter: @ZLeeGU at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


