San Francisco, California (UroToday.com) For a case-based discussion assessing real-world challenges in urothelial carcinoma, Peter Clark, MD, and Srikala Sridhar, MD, MSc, FRCPC, assembled a panel that included Drs. Brian Baumann, Andrea Apolo, Alison Birtle, and Nicholas James.
Case 1:
This patient was a 52-year-old woman, who was otherwise healthy and presented with hematuria. The TURBT showed a high-grade urothelial carcinoma with muscle invasion; an exam under anesthesia suggested cT3 disease and there was a solitary 2 cm external iliac node on imaging. The CT chest/abdomen and pelvis were otherwise unremarkable and the patient is now asymptomatic. Options at this point include:
- Cisplatin-based neoadjuvant chemotherapy (6 cycles) + cystectomy + pelvic lymph node dissection
- Cisplatin-based neoadjuvant chemotherapy (4 cycles) + cystectomy + pelvic lymph node dissection
- Cystectomy + pelvic lymph node dissection
- Concurrent chemoradiotherapy
- Immune checkpoint inhibitor
Dr. Apolo notes that given the fact there is a lymph node involved she would opt for 6 cycles of chemotherapy rather than 4 cycles. Dr. Birtle also notes that there likely is a role of neoadjuvant chemotherapy followed by radiosensitizing chemotherapy at the time of radiation therapy if the patient chose to not have a cystectomy. Dr. Apolo highlights that the AJCC staging has changed with the new 8th edition. Previously, the 7th edition would classify any pelvic lymph (1-3) or T4b alone as stage 4, whereas in the 8th edition T4a disease is stage 3a, T4b is stage 4a, N1 is stage 3a, N2/3 is stage 3b, and M1a (distant lymph node) is stage 4a. With regards to type of chemotherapy, Dr. Apolo notes that with the new staging system this patient would be a stage 3a and she would opt for 3-4 cycles of dd-MVAC neoadjuvant chemotherapy over ~9 weeks for this patient.
The patient went on to have 3 cycles of chemotherapy with a disease response and ended up completing all 6 cycles of chemotherapy. She then had a radical cystectomy with pelvic lymph node dissection and pathology showed residual high-grade disease with node positivity. According to Dr. Baumann, there is rationale for adjuvant radiotherapy for locally advanced disease, which includes:
- High rates of local-regional failure for pT3-4 N0-N+ disease (20-41% at 5 years) from modern prospective cystectomy series
- Chemotherapy did not reduce the risk of local-regional failure in SWOG 8710 or EORTC/MRC trials
- Local-regional failure is rarely salvageable and is associated with poor median survival (9 months)
- Modern radiotherapy techniques can reduce side effects, which discouraged previous attempts with adjuvant radiotherapy in the ‘70s and 80’s
An International Consensus Contouring Guidelines created by a multidisciplinary expert team (5 countries, 15 institutions) recommended that for patients with negative margins, the clinical targets should be the pelvic lymph nodes alone. If there were positive margins, then the recommended clinical target for radiation would be the pelvic lymph nodes and the cystectomy bed. This guideline panel also recommended that IMRT was mandated to reduce the dose to the bowel and urinary diversion, and the standard dose per GETUG, NRG, and NCCN was 50.4 Gy in once daily fractions. Furthermore, the late grade 3 GI toxicity on modern trials is low at 7%. With regards to systemic therapy in this setting, the phase III AMBASSADOR trial will assess adjuvant pembrolizumab in muscle invasive and locally advanced urothelial carcinoma vs observation. Patients will be randomized to pembrolizumab 200 mg q3 weeks for one year vs observation with a co-primary endpoint of OS and DFS.
Case 2:
This patient was a 72-year-old man with a medical history of hypertension, an ex-smoker, lives in supportive living with an ECOG performance status of 2. He was found to have a high-grade, bulky tumor with hydronephrosis. Dr. Birtle notes that this patient highlights the importance of a frailty screen with G8 and Mini-Cog. Based on the results of these metrics the patient is then classified as fit, vulnerable or frail. According to Dr. Birtle there are also a number of items we can assess to see if patients will tolerate systemic therapy, included in this algorithm (the CRASH score) is: diastolic BP, ADLs, LDH, ECOG PS, MMS, MNA and Chemotox score. She also highlighted that if this patient were to go on and have a cystectomy we should focus on ERAS pathways to improve this patient’s recovery. Dr. James notes that chemoradiation in the UK is much different than the US in that a patient with hydronephrosis in the US would be excluded from TMT, whereas in the UK hydronephrosis would be regarded as a prognostic factor, not an exclusion criteria.
This patient then underwent a TURBT and concurrent chemoradiation. Dr. James notes that the presence of a residual mass is highly correlated with extent of resection: 96% complete resections without residual mass and 66% incomplete resections have residual mass. Dr. James also highlighted that quality of life data for patients undergoing chemoradiation did not differ compared to those undergoing chemotherapy alone. There are several first line trials of PD1/PDL1 targeting ongoing which are coupled with concomitant radiotherapy for patients with MIBC: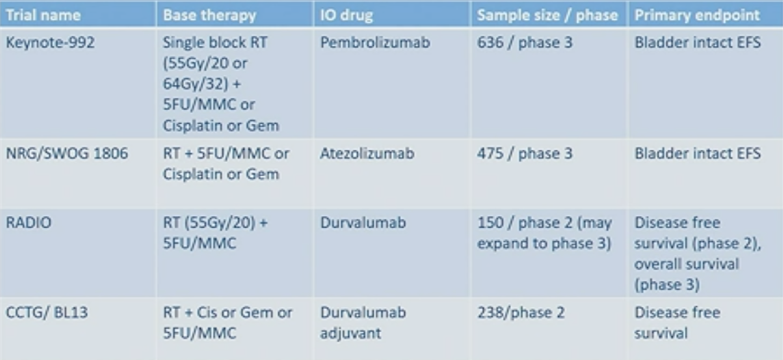
Dr. Sridhar then commented regarding what if this patient were frail with a history of prior brachytherapy for prostate cancer? Are there symptoms to palliate (current urinary function, balance local control and symptoms, maximal TURBT and systemic therapy)? Furthermore, if he proceeds with radiotherapy, is this to palliate symptoms and would it consist of 21 Gy in 3 fractions? Dr. Birtle states that it is necessary to have a detailed list of current symptoms and if the patient has poor urinary function at the outset, it is likely that further radiotherapy will make these symptoms worse. She would maximize with a TURBT first and then assess candidacy for IO therapy, which has less side effects than chemotherapy. She would go ahead with 21 Gy with 3 fractions.
Case 3:
This patient was a 77-year-old man with medical history of diabetes for 25 year, hypertension and hyperlipidemia, ex-smoker, lives alone and developed hematuria in 2012. He was diagnosed with right upper tract urothelial carcinoma and underwent a right nephroureterectomy (pT2N0). Dr. Birtle notes that this patient should be considered for adjuvant chemotherapy based on findings of the POUT trial randomizing patients to adjuvant chemotherapy vs surveillance. The proportion of event free patients at 2 years was 0.71 (95% CI 0.60-0.79) for chemotherapy compared to 0.54 (95% CI 0.43-0.64) for surveillance. After adjustment for nodal involvement, microscopic margin status and planned chemotherapy type, chemotherapy resulted in a significantly improved DFS (HR 0.47, 95% CI 0.30-0.74; p = 0.001). Furthermore, the DFS effects were seen across all subgroups.
In 2014 the patient then presented with a new right flank mass and biopsy confirmed metastatic urothelial carcinoma. He went on to receive three cycles of gemcitabine + cisplatin chemotherapy, which he tolerated poorly and there was no response on staging. Dr. Baumann notes that he would favor local therapy in this situation given that the potential is there for cure with local therapy. Subsequently he underwent surgery uneventfully, which included a 6 x 8 cm mass, 0/6 lymph nodes were positive and margins were negative. In September 2019, he presented again with a right flank mass (5 cm) and two new liver metastases. At this point Dr. Baumann favors that this patient may be a candidate for SBRT based on data published last year in the SABR-COMET trial. Dr. James agrees that SBRT is generally well-tolerated and would not preclude the patient from receiving subsequent chemotherapy. If there was no local therapy, what systemic therapy would be available? Dr. Apolo highlighted that we now have novel systemic targeted therapies, namely erdafitinib, which is an FGFR inhibitor in patients with metastatic urothelial carcinoma and FGFR alterations. With erdafitinib, the response rate was 40%. We also have enfortumab vedotin, an antibody conjugate targeting nectin-4, and in a phase 1 study in patients with metastatic urothelial carcinoma, enfortumab vedotin also had a 40% response rate among patients that had progressed after chemotherapy or immunotherapy. Dr. Apolo feels that combination therapy is likely the way forward – quoting data presented at this meeting, the combination of enfortumab vedotin plus pembrolizumab had an incredible 73% response rate in cisplatin ineligible patients in this phase I study. Both erdafitinib and enfortumab vedotin have received FDA approval.
Chairs of the Panel:
Peter E. Clark, MD, Levine Cancer Institute, Atrium Health, Charlotte, NC
Srikala S. Sridhar, MD, MSc, FRCPC, Princess Margaret Hospital, Toronto, ON, Canada
Panelists:
- Radiation Oncologist: Brian C. Baumann, MD, Washington University, St. Louis, MO
- Medical Oncologist: Andrea B. Apolo, MD, National Cancer Institute, Bethesda, MD
- Clinical Oncologist: Alison J. Birtle, Lancashire Teaching Hospitals NHS Foundation, UK
- Clinical Oncologist: Nicholas D. James, MBBS, PhD, The Institute of Cancer Research, The Royal Marsden Hospital, UK
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


