San Francisco, CA (UroToday.com) At this session — revived after a one year hiatus in 2019 — Aly-Khan A. Lalani, MD, FRCPC, provided his perspectives on the most impactful publications in renal cell carcinoma from 2019. He first reviewed a timeline of advancements in front-line treatment options in renal cell carcinoma (RCC). These were notable for interferon and high-dose IL-2 treatment in the 1980s and 1990s, which first pointed to the significance of the immune system in this disease. In the first two decades of the 2000s, many advances were made including sunitinib, bevacizumab and interferon, temsirolimus +/- interferon, pazopanib, tivozanib, cabozantinib and nivolumab + ipilimumab.
The studies in this Best of Journals: RCC update are notable for synergistic strategies with immunotherapy involving tyrosine kinase inhibitor (axitinib and bevacizumab). The studies discussed were:
- Javelin Renal 101 (avelumab and axitinib)
- Keynote-426 (pembrolizumab and axitinib)
- CheckMate-214 (42 month update of nivolumab/ipilimumab)
- Atezolizumab and bevacizumab in variant histology RCC
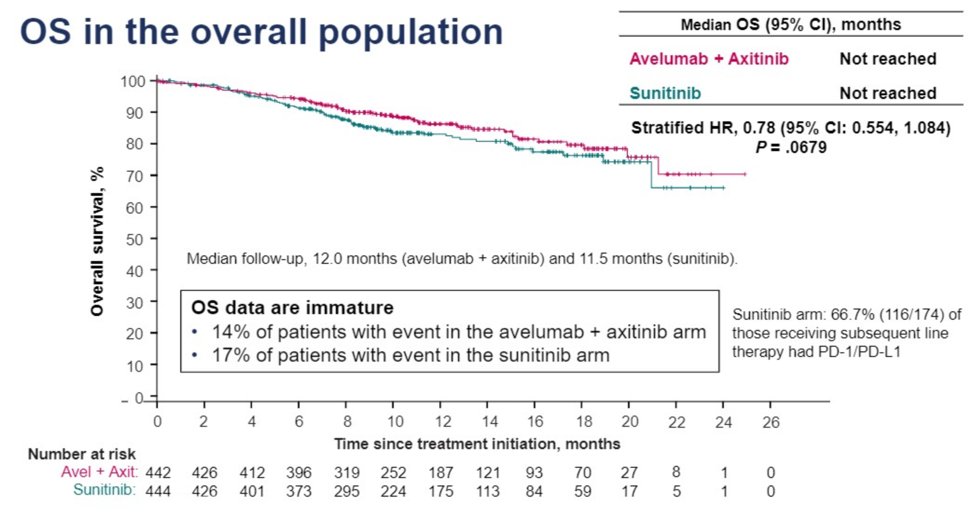
This was a first-line study for advanced renal cell carcinomas that randomized patients in a 1:1 ratio to either sunitinib as the control arm or the tyrosine kinase inhibitor axitinib in combination with the anti-PDL1 antibody avelumab. The primary endpoints of the study were progression free and overall survival in patients who were PD-L1 positive as documented by > 1% of immune cells staining positive by the Ventana SP263 assay. The randomization scheme is shown below.
Of the patient enrolled, around 20% of patients were favorable risk, which is typical for patients receiving their first-line therapy for advanced disease. The study showed a statistically significant improvement in median progression free survival from 8.4 months with sunitinib alone to 13.8 months with axitinib + avelumab (HR for progression or death 0.61, 95% CI 0.47-0.79, p < 0.001). The overall response rate was 51% in the intervention arm and was slightly higher at 55.2% amongst PD-L1 positive patients. At a median of twelve months of follow-up there was a visual trend towards improved overall survival but this was not stastically significant (p = 0.0679). Two-thirds of patients randomized to sunitinib therapy went on to receive subsequent PD1 axis therapy.
Almost all patients in both arms experienced adverse events, with just over 70% of events being grade 3 above in both arms. In the intervention arm, 11% of patients required steroids for the management of immune related adverse events.
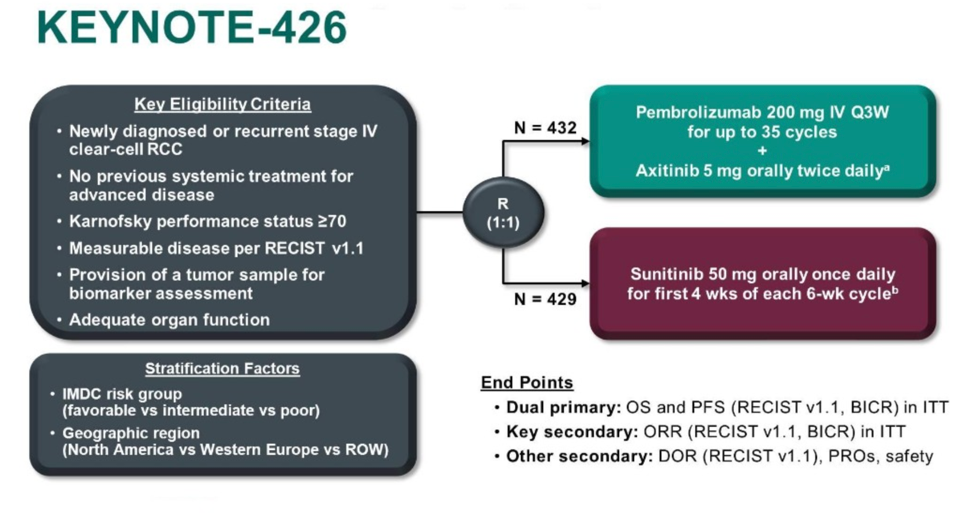
This was a study with a similar intervention to Javelin Renal 101 except pembrolizumab was utilized as the immunotherapy agent. Patients were randomized in a 1:1 fashion to either the control arm of sunitinib monotherapy or the intervention arm of axitinib plus pembrolizumab. The primary endpoints were progression free and overall survival, with overall response rate and duration of response as well as safety and patient reported outcomes as secondary endpoints.
Relative to Javelin Renal 101, 30% of patients were favorable risk by IMDC classification, which is somewhat higher than expected from the general population. With a median follow-up of 12.8 months, the intervention arm had an improved overall survival rate (89.9% alive versus 78.3% alive, HR for death 0.53, 95% CI 0.38-0.74, p < 0.001) and improved progression free survival (15.1 months versus 11.1 months, HR 0.69, p < 0.001). The overall response rate was 59.3% in the intervention group. Benefit was seen across IMDC risk group regardless of PD-L1 expression. 62% of patients randomized to sunitinib went on to receive PD1 axis therapy.
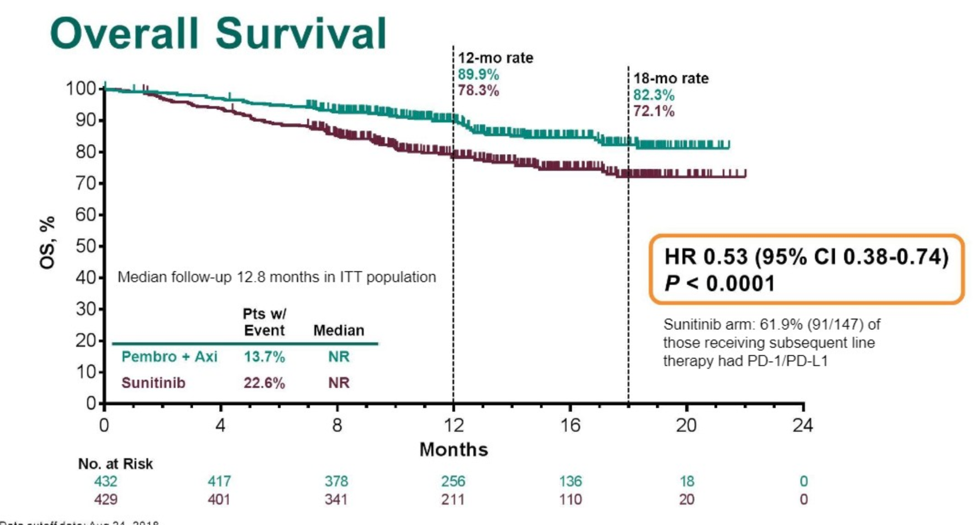
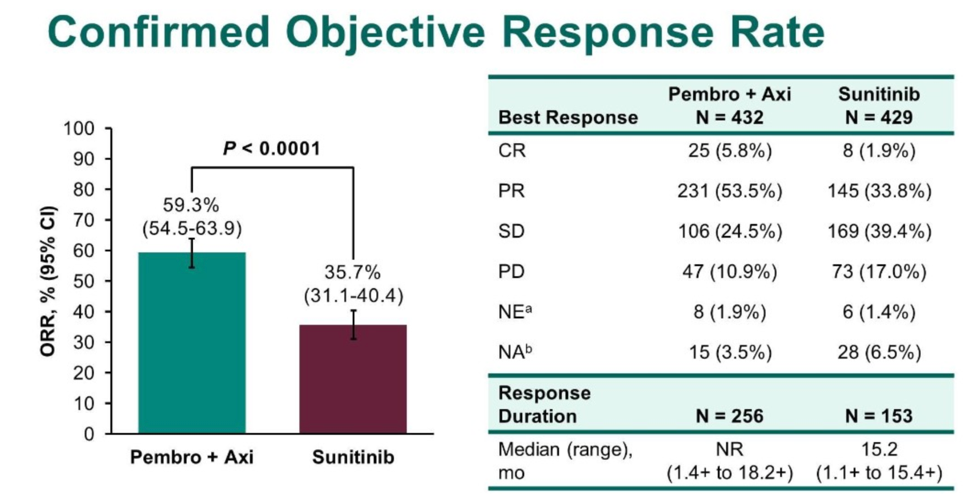
It is interesting to note the cohort in Keynote-426 had a higher proportion of favorable risk patients, which was also reflected by the overall longer progression-free survival median interval.
Published in 2019, and again updated at GU ASCO 2020, longer-term follow-up from the CheckMate 214 trial exploring nivolumab and ipilimumab versus sunitinib as first line therapy in intermediate/poor risk advanced RCC continue to show an overall survival advantage for combination immunotherapy. Importantly, a proportion of patients with this therapy have sustained complete responses. This benefit does not seem to extend to IMDC favorable risk patients.
Based on these three trials and other published data, Dr. Lalani offered the following table.
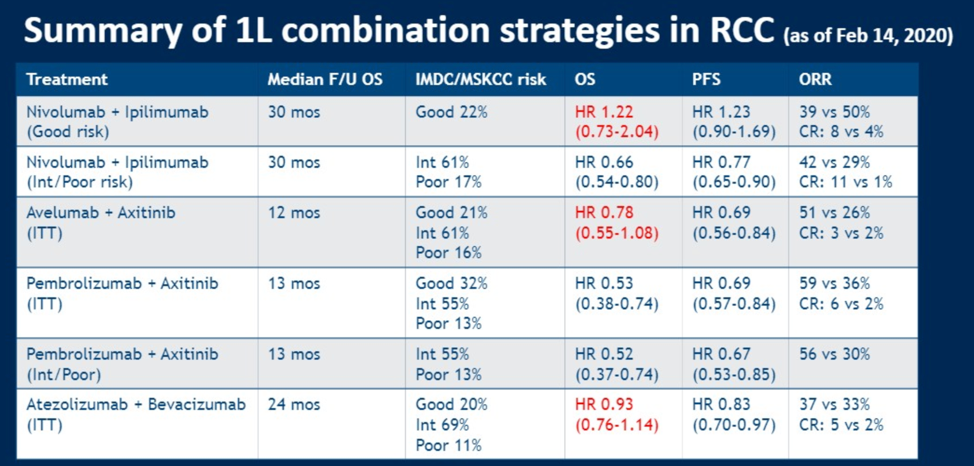
These data have largely been incorporated into treatment guidelines, as shown by a snapshot of the current kidney cancer NCCN guidelines.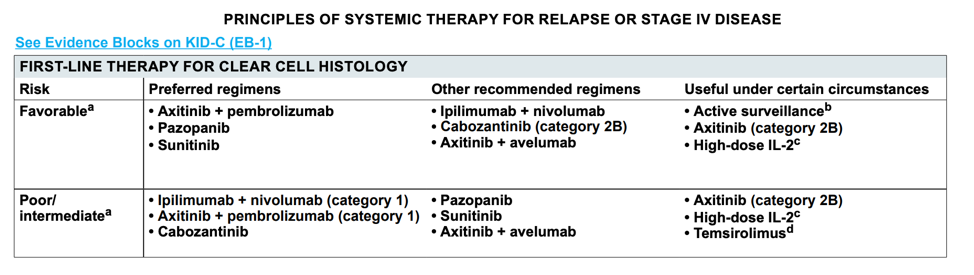
The treatment of renal cell carcinomas with variant histologies remains a clinical challenge. Dr. Lalani discussed the publication of a phase II study of the anti-PDL1 antibody atezolizumab in combination with the anti-VEGF antibody bevacizumab.
As shown in the results slide, the overall response rate was 33%, with response rate of up to 50% in patients with clear cell RCC. Of the variant histologies included, chromophobe RCC had an especially poor response rate.
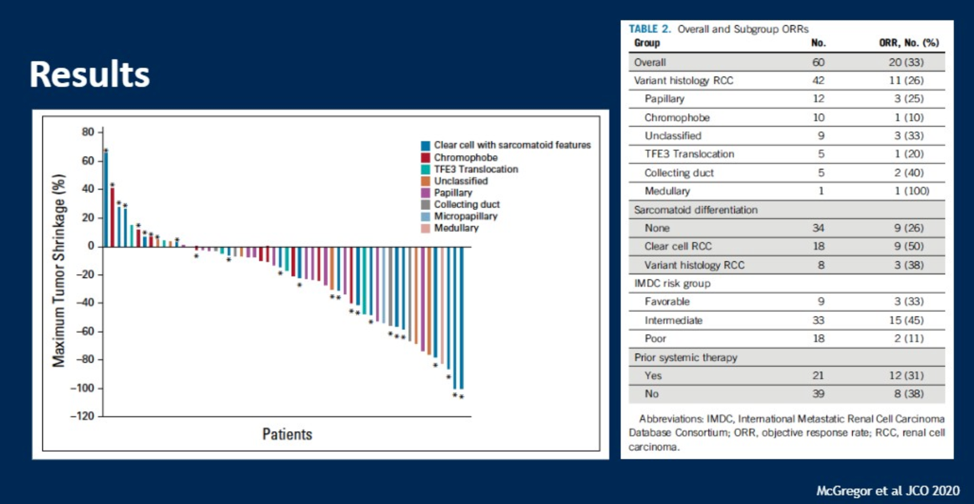
Patients found to be positive for PD-L1 expression had higher response rates relative to non-PDL1 positive patients.
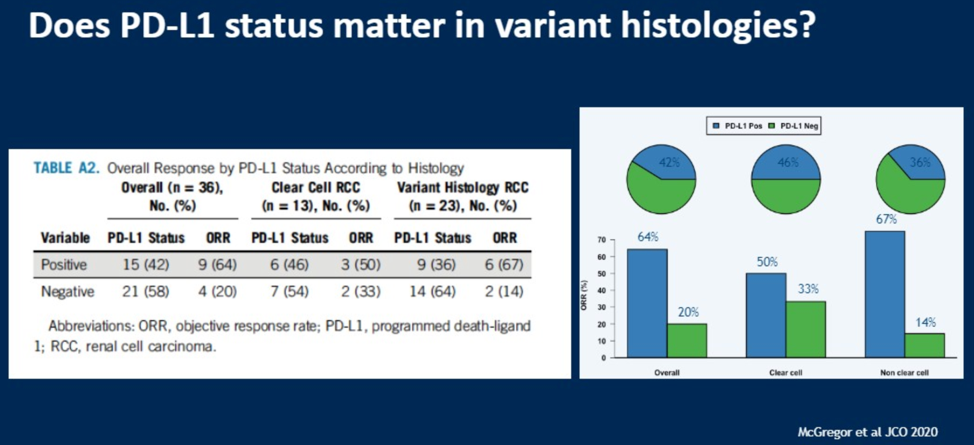
While promising, significantly more work is required in these variant histology patients to improve their outcomes.
Upcoming Trials
Finally, Dr. Lalani discussed earlier stage trial data that is emerging and was also presented this year at GU ASCO. He highlighted the phase 1 / 2 trial of HIF-2alpha inhibition with MK-6482 that is promising and will be evaluated in a phase 3 trialed as compared with everolimus.
In conclusion, Dr. Lalani discussed that there are multiple combination options in the first line therapy of advanced renal cell carcinoma, and the decision of which regimen to use is based on the patient and potential side effect profiles. Upcoming trials in the first-line space include CheckMate 9ER (nivolumab + cabozantinib) and CLEAR (lenvatinib/everolimus versus lenvatinib/pembrolizumab versus sunitinib). Triplet strategies are also under investigation. Non-clear cell histologies remain an area where further clinical development is an unmet clinical need.
Presented by: Aly-Khan A. Lalani, MD, FRCPC, Medical Oncologist at the Juravinski Cancer Centre, McMaster University, Canada
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 ASCO Genitourinary Cancers Symposium (#GU20), February 13th-February 15th, San Francisco, CA


