Sitravatinib is an orally-available small molecule, multi-targeted TKI. Inhibition of targets such as TAM family, Split family and c-MET is thought to enhance anti-tumor activity through depletion of immunosuppressive cell populations (for example, MDSCs and regulatory T cells) and increasing T cell priming. In a RCC expansion cohort from a phase IB trial of single-agent sitravatinib, a promising 25% conformed objective response rate was observed with 94% achieving clinical benefit and durable responses noted.
The hypothesis of this study was that sitravatinib could augment nivolumab responses in accRCC. To this end, the study’s primary endpoint was both safety and efficacy (absence of progression within 6 weeks). The patient population included a similar eligibility criteria to CheckMate 025, histologically-confirmed clear cell TCC with disease progression after 1-2 prior lines of TKI therapy and measurable disease. Dose-finding was performed using the sequentially adaptive phase I-II late-onset EffTox strategy. Of note, numerous correlative studies were built into the study design.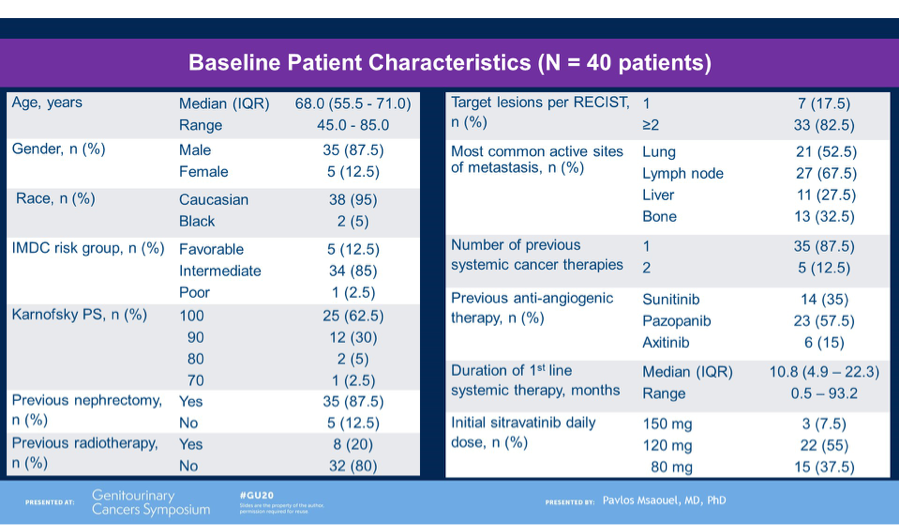
Dr. Msaouel detailed the study cohort of 40 patients, who were predominantly male and intermediate-risk for IMDC criteria. Of note, most patients had 1 prior line of cancer therapy (mostly sunitinib or pazopanib).
Nivolumab plus sitravatinib showed promising efficacy, with 15/38 (39%) achieving a confirmed objective response and 35/38 (92%) achieving clinical benefit (stable disease or partial response or completed response). Put into historical context, this is promising anti-tumor activity compared to the rate of response of single agents in this setting, such as nivolumab (35%), cabozantinib (17-21%). Furthermore, at a median follow-up of 17.7 months, median overall survival had not been reached with 79% of patients alive. Median duration of treatment (10.3m) compared favorably to historical report of nivolumab alone (4.6 months).
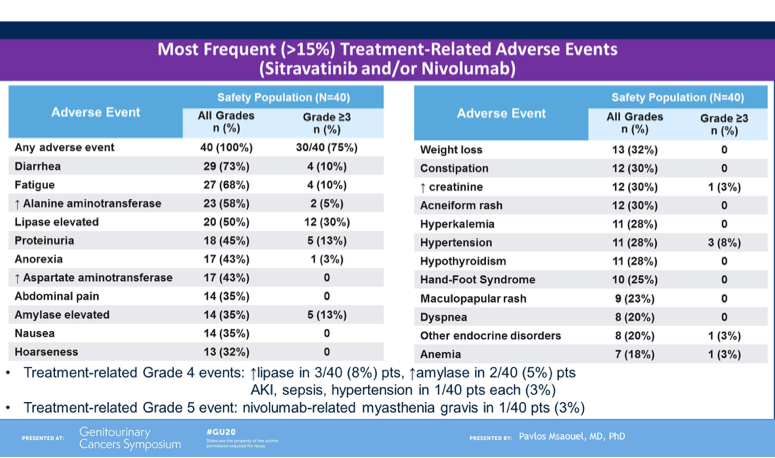
Lastly, Dr. Msaouel noted the safety profile of the combination. Most frequent adverse events included diarrhea, fatigue, liver function test derangement, lipase elevation and proteinuria. The most common high-grade toxicities included lipase/amylase elevation, proteinuria and fatigue. In total 4/40 (10%) patients discontinue treatment due to adverse events. Approximately half the cohort required dose-reduction of sitravatinib due to toxicity, and this occurred mostly in the first 12 weeks. A dose of 120mg is now being explored in other tumor types in combination with nivolumab.
In summary, this phase I/II study of sitravatinib, a multi-targeted oral TKI, in combination with nivolumab in pre-treated accRCC, showed higher objective response rate and longer PFS than historically reported single-agent nivolumab in this setting. Further studies, including patient-reported outcomes and biological correlatives are underway. This combination represents a promising strategy, however, given that PD-1 inhibition (in combination) is standard 1st-line treatment for this patient population, the role of the combination of sitravatinib plus nivolumab after progression on prior PD-1 inhibitor therapy remains less clear and more data is required.
Presented by: Pavlos Msaouel, MD, PhD, Medical Oncologist, MD Anderson Cancer Center, Houston, TX
Written by: Anis Hamid, MBBS, Medical Oncology Research Fellow at Dana-Farber Cancer Institute and Medical Oncologist, PhD candidate, University of Melbourne, Australia (Twitter: @anis_a_hamid) at the 2020 ASCO Genitourinary Cancers Symposium (#GU20), February 13th to 15th, 2020, San Francisco, CA.


