Dr. Lucia Nappi offered a case illustrating the need for biomarkers. The case was of a 40-year-old man who was found to have a left testicular mass. Orchiectomy revealed a left 9 cm seminoma. Pre-orchiectomy markers were notable for an LDH of 248 and AFP of 9.4, both of which normalized after surgery. A baseline staging CT showed a single 13 x 15 mm periaortic lymph node located in the potential landing zone for the tumor. A follow-up CT scan showed mild enlargement of the same node 10 weeks later. Because the patient was adamantly opposed to adjuvant therapy unless absolutely indicated, a biopsy was performed. This identified follicular lymphoma in the lymph node. Dr. Nappi suggested that a biomarker could have helped avoid the biopsy as well as the treatment uncertainty in this patient.
She then went on to describe published work identifying microRNA 371a-3p (miR371) as a biomarker in testicular germ cell tumors. MicroRNAs are short (20-23 nucleotide) long segments of non-coding RNAs that regulate gene expression. miR371 is highly expressed in 90% of germ cell tumors including both seminomas and non-seminomas, but not teratomas. Its expression is specific to viable germ cell tumors (and only otherwise expressed in pregnancy), and it is detectable in small amounts of plasma. Finally, its expression decreases rapidly after treatment with a half-life of 16 hours.
The validity of miR371 in germ cell tumors has been shown in numerous studies, summarized below.
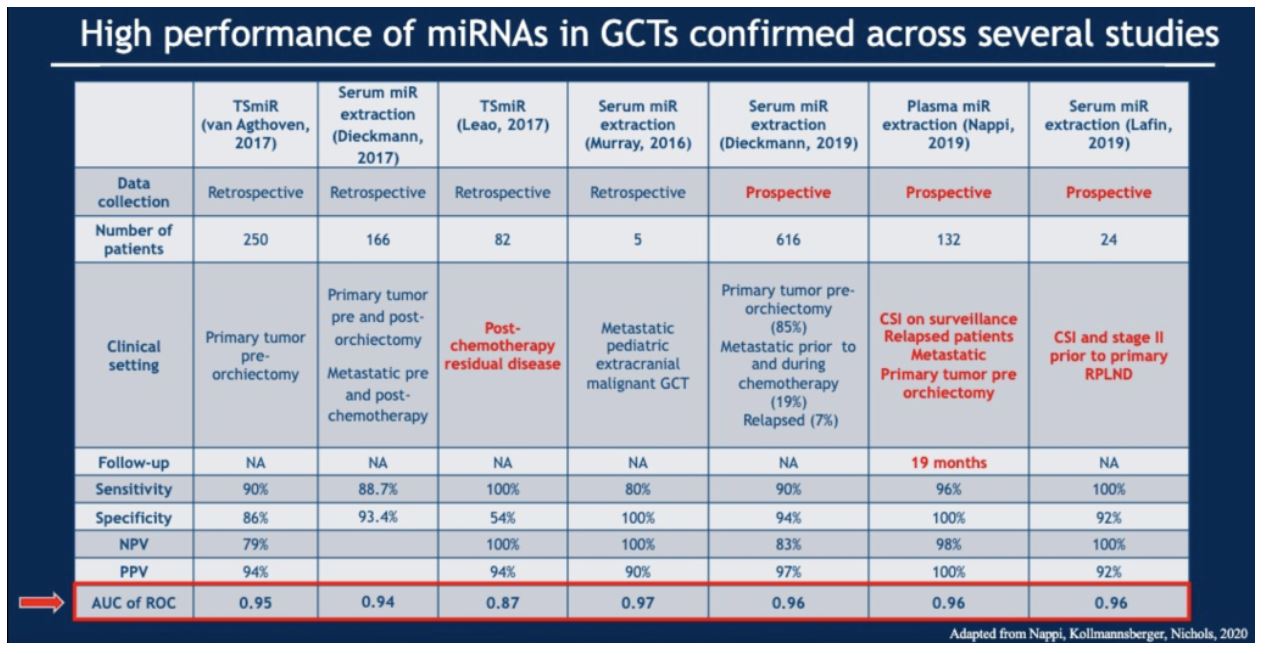

The available data suggest that miR371 is a highly sensitive and specific test, is easily measurable, is low cost ($60 per test), reproducible and non-invasive. Together, these suggest that it may be an ideal biomarker. However, Dr. Nappi suggests that it is not quite ready for primetime given that its clinical utility has not been prospectively proven.
To this end, several trials are underway to evaluate miR371 prospectively. This includes SWOG1823, which examines the question of whether miR371 can be used to detect tumor relapse in patients with clinical stage 1 or 2A disease after orchiectomy. Additionally, the miRNARx study will explore whether miR371 expression can guide the reduction of therapy in patients who have recurrence of their clinical stage 1 germ cell tumor.

Dr. Nappi then proposed a potential set of clinical applications for miR371, should its clinical utility be proven.

In summary, miR371 is a biomarker with proven clinical and analytical validity for non-teratoma testicular germ cell tumors. Its clinical utility for impacting treatment and patient outcomes is currently unknown, but at least two studies are ongoing to partially address this question. Additionally, other microRNA biomarkers such as miR375 are being evaluated for their utility in teratoma.
Presented by: Lucia Nappi, MD, PhD, Medical Oncologist and Senior Research Scientist, BC Cancer Agency, British Columbia, Canada
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2020 ASCO Genitourinary Cancers Symposium (#GU20), February 13th-February 15th, San Francisco, CA


