San Francisco, California (UroToday.com) Approximately 90% of patients with mCRPC exhibit bone metastases, which are associated with an increased rate of fractures, spinal cord compression, bone surgery, and external beam radiotherapy. Radium-223 is a targeted alpha therapy that accumulates in areas of increased bone turnover surrounding metastatic lesions and prolongs the OS of patients with mCRPC.1 Prospective, randomized clinical trial data comparing treatment sequences and combinations in mCRPC are currently limited. The phase III ERA 223 study assessed the efficacy and safety of radium-223 in combination with abiraterone plus prednisone or prednisolone in patients with CRPC and bone metastases, demonstrating an increased frequency of bone fractures with radium-223 plus abiraterone and prednisone/prednisolone versus placebo plus abiraterone and prednisone/prednisolone.2 At the prostate cancer poster session at GU ASCO 2020, Dr. Neal Shore and colleagues reported results of real-world symptomatic skeletal events and overall OS of patients with mCRPC who received concurrent or layered Radium-223 plus enzalutamide or abiraterone/prednisone.
This study included patients with mCRPC treated with Radium-223 in US cancer clinics from January 1, 2013 to June 30, 2017 identified from a Flatiron prostate cancer registry of electronic health records. Treatment initiation defined subgroups included: concurrent (both started within 30 days) or layered (one drug started ≥30 days after the other). The baseline was the first dose of Radium-223. Descriptive analyses were conducted on the baseline characteristics, prior therapies, bone health agent (denosumab or bisphosphonates) use, and clinical outcomes including symptomatic skeletal event rates and OS. The Kalpan-Meier method was used for time-to-event analyses (OS).
There were 625 patients treated with Radium-223, of which 303 (48%) received Radium-223 plus enzalutamide or abiraterone/prednisone. Layered treatment was more common (73%) than concurrent (27%) treatment. The median follow-up time was 9 months in the main cohort. Median OS from mCRPC diagnosis was 28.3 months (95% CI 18.4 to not reached) in patients who received concurrent Radium-223 plus abiraterone/prednisone and 34.5 months (95% CI 25.9-50.9) in those who received layered Radium-223 plus abiraterone/prednisone. Median OS from mCRPC diagnosis was 28.1 months (95% CI 16.7 to not reached) in patients who received concurrent Radium-223 plus enzalutamide and 26.9 months (95% CI 25.0-34.4) in those who received layered Radium-223 plus enzalutamide.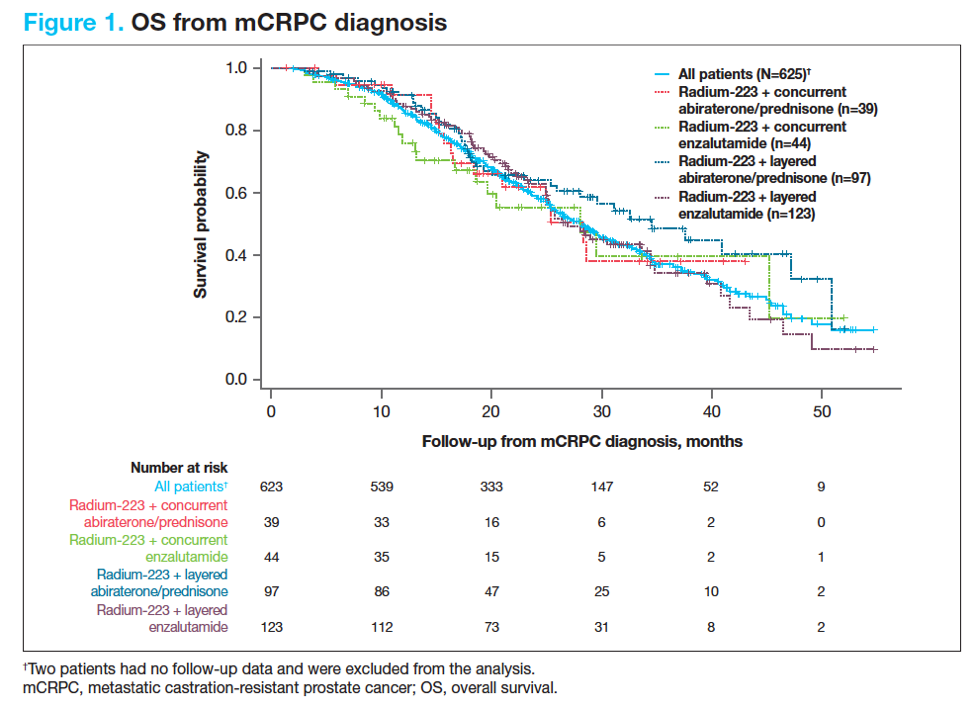
Concomitant use of any bone health agents occurred in nearly 2/3 of patients and symptomatic skeletal events and fracture rates stratified by bone health agents is as follows:

This study concluded that Radium-223 plus enzalutamide or abiraterone/prednisone treatment was mainly layered and that ~50% of patients with mCRPC in a real-world setting was receiving additional agents with Radium-223. Symptomatic skeletal event rates with layered vs concurrent Radium-223 plus abiraterone/prednisone varied between subgroups. These results must be treated cautiously given the small patient numbers and a non-randomized study. The ongoing PEACE III trial is investigating concurrent Radium-223 plus enzalutamide and a Phase III study (ESCALATE) exploring layered Radium-223 plus enzalutamide is planned.
Clinical trial information: NCT02043678
Presented by Neal Shore, MD, FACS, Medical Director for the Carolina Urologic Research Center, Myrtle Beach, South Carolina, USA.
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019 Mar;20(3):408-419.


