Dr. Julie Graff presents data from the remaining two cohorts (Cohort 4, C4; Cohort 5, C5). Notably, cohorts 1-3 received pembrolizumab monotherapy. In contrast, C4 and C5 had pembrolizumab added to enzalutamide, as combination therapy. Prior single-institution experiences in a phase II study (n = 28) demonstrated favorable efficacy with the addition of pembrolizumab to enzalutamide after progression on enzalutamide, lending clinical support for combination therapy3. All subjects in cohorts 4 and 5 had previously responded to enzalutamide but with subsequent progression. PD-L1 status was mixed and reported in the authors’ Table 1. Patients were enrolled irrespective of prior abiraterone exposure. All patients had ECOG PS of 0-2 and were chemotherapy-naïve.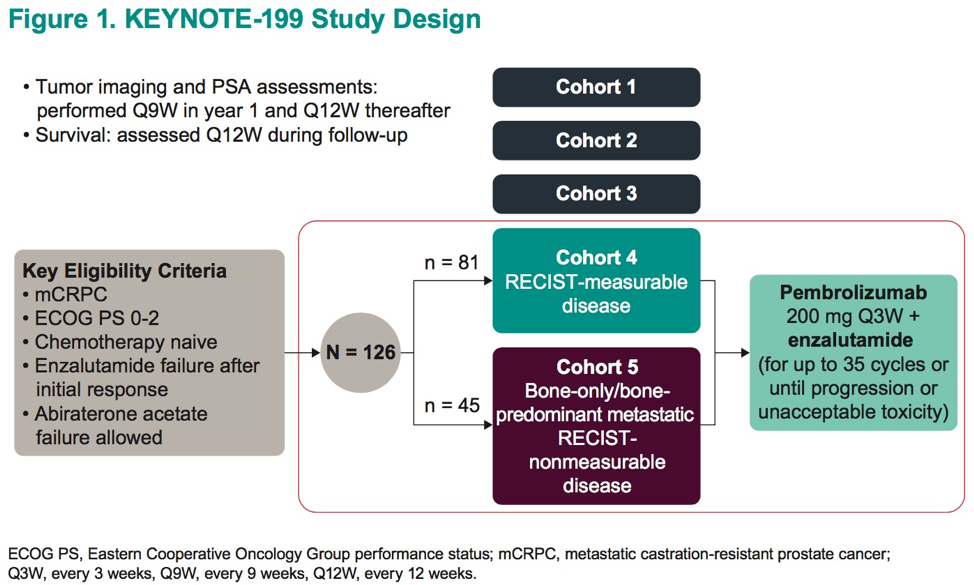
C4 (n = 81) subjects were men with measurable disease and C5 (n = 45) had bone-predominant disease. All patients received pembrolizumab at flat dosing (200 mg q3w) in addition to continuation of enzalutamide for up to 35 cycles or until either disease progression or intolerable toxicity.
The primary endpoint was ORR via RECIST via blinded independent central review for C4. Secondary endpoints included disease control rate (DCR), PSA RR (≥50% reduction), radiographic progression-free survival (rPFS), OS, and safety. Duration of response was including for C4.

At a median time of follow-up of 13.7 months (C4, 11.8 mos; C5 18.6 mos), 107 of 126 patients had discontinued pembrolizumab, most due to disease progression. ORR for patients with measurable disease (C4) was 12% (95% CI 6-11). Highlighted within the responders were 2 complete responses (of 8 responses) as well as a duration of response of 6 months in 60% of those patients whose tumors responded. Additionally, responses after cessation of pembrolizumab were observed. Reported DCR for all patients were 51% in C4 (95% CI 39-63) and 51% in C5 (95% CI 36-66).
With regard to safety, All Grade (C4 75%, C5 69%) and Grade 3-5 (C4 26%, C5 24%) adverse events (AEs) were similar as compared to cohorts 1-3 but numerically more frequent. The authors note that rash was more commonly experienced by subjects than has been previously reported for either agent alone. Whether this reflects the intrinsic additive likelihood of experiencing an AE from two separate drugs simultaneously or some interaction of the two mechanisms is unknown.
These data demonstrate clinical support for the preclinical rationale for the addition of the androgen receptor inhibitor enzalutamide to immune checkpoint blockade with pembrolizumab. Again, as in prior cohorts of KEYNOTE-199 and in other disease types, responses are in a minority of patients, but many responding patients experience a durable response. It is anticipated that use of additional clinical, pathological, or genomic parameters could help identify those patients most likely to respond to pembrolizumab. The combination of enzalutamide to pembrolizumab appears to demonstrate higher ORR in those patients with measurable disease as compared to pembrolizumab alone (Cohorts 1-2), supporting the ongoing phase III clinical trial of this combination in enzalutamide-naïve patients (KEYNOTE-641, NCT03834493)4.
Presented by: Julie N. Graff, MD, OHSU Knight Cancer Institute, Portland, Oregon
Written by: Jones Nauseef MD, Ph.D. Fellow, Division of Hematology and Oncology, Weill Cornell Medicine/New York-Presbyterian Hospital. Twitter: @DrJonesNauseef at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Clinical Trial Information: NCT02787005
2. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. “Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study.” 2019. J Clin Oncol. 38: 395-405.
3. Graff JN et al. J Clin Oncol. 2018;36(suppl). Abstract 5047
4. Clinical Trial Information: NCT03834493


