In the ENZAMET study, men with mHSPC were randomly assigned 1:1 to receive testosterone suppression plus either enzalutamide or a non-steroidal anti-androgen.1 Randomization was stratified by: volume of disease (high vs low, according to CHAARTED), planned early docetaxel, planned anti-resorptive therapy, comorbidity score (ACE-27), and study site. The primary endpoint was overall survival. Secondary endpoints were prostate-specific antigen (PSA) progression-free survival (PFS), clinical progression-free survival, and adverse events. Subgroup analyses to assess possible modulation of the treatment effect were specified a priori and included planned early docetaxel (yes vs no) and volume of disease (high vs low). ENZAMET randomly assigned 1,125 patients: 562 in the non-steroidal anti-androgen and 563 in the enzalutamide arm. Overall survival was significantly prolonged in the enzalutamide arm compared to the non-steroidal anti-androgen arm (hazard ratio [HR] 0.67, 95% confidence interval [CI] 0.52-0.86). Furthermore, time to PSA rise, clinical progression or death (HR 0.39, 95% CI 0.33-0.47) and time to clinical progression (HR 0.40, 95% CI 0.33-0.49) both favored enzalutamide. In the prespecified subgroup analysis of docetaxel (yes vs no), enzalutamide significantly improved time to clinical progression among men receiving docetaxel (HR 0.48, 95% CI 0.37-0.62), but did not improve overall survival (OS) (HR 0.90, 95% CI 0.62-1.31). In men not receiving docetaxel, enzalutamide improved clinical progression (HR 0.34, 95% CI 0.26-0.44) and OS (HR 0.53, 95% CI 0.37-0.75). Dr. Morgans notes that there are results still to come, including quality of life analyses by patient-reported outcomes, health economics analyses, translational biological studies, and long-term survival data.
The TITAN study was a Phase III, double-blind, randomized study designed to determine whether apalutamide, a selective next-generation androgen receptor inhibitor, plus androgen deprivation therapy (ADT) improves radiographic progression-free survival (rPFS) and OS compared with placebo plus ADT in men with metastatic castration-sensitive prostate cancer (mCSPC).2 Dual primary endpoints were rPFS and OS. Secondary endpoints were time to (i) initiation of cytotoxic chemotherapy, (ii) pain progression, (iii) chronic opioid use, and (iv) skeletal-related event. Patients were randomized 1:1 to apalutamide (240 mg/d) or placebo, added to ADT, in 28-day cycles. There were 525 patients randomized to apalutamide and 527 to placebo. The median age was 68 years, 8% had prior treatment for localized disease, and 11% had prior docetaxel. With regard to disease burden, 63% had high-volume disease and 37% had low-volume disease. At a median follow-up of 22.6 months, 66% of patients on apalutamide and 46% of patients receiving placebo remained on treatment. Apalutamide significantly improved rPFS (HR 0.48, 95% CI 0.39-0.60), with a 52% reduction in risk of death or radiographic progression. Importantly, this benefit was observed across all subgroups analyzed. Median rPFS was not reached in the apalutamide group and was 22.1 months in the placebo group. Second, apalutamide significantly improved OS (HR 0.67, 95% CI 0.51-0.89), with a 33% reduction in the risk of death. Median OS was not reached in the apalutamide or placebo group. As of now, Dr. Morgans notes that we now have four category 1 options for systemic treatment for mCSPC.
The STAMPEDE trial recently assessed the long-term survival results based on low- and high-burden metastatic disease.3 Metastatic burden was assessable for 830/1086 (76%) patients: 362 (44%) had low and 468 (56%) high metastatic burden. Median follow-up was 78.2 months. There were 494 deaths on the standard of care arm (41% more than the previous report). Furthermore, there was good evidence of a benefit of docetaxel over the standard of care on OS (HR = 0.81, 95% CI 0.69-0.95, p = 0.009) with no evidence of heterogeneity of docetaxel effect between metastatic burden sub-groups (interaction p = 0.827). Analysis of other outcomes found evidence of benefit for docetaxel over standard of care in failure-free survival (HR = 0.66, 95% CI 0.57-0.76, p < 0.001) and PFS (HR = 0.69, 95% CI 0.59-0.81, p < 0.001) with no evidence of heterogeneity of docetaxel effect between metastatic burden sub-groups (interaction p > 0.5). Based on these results, the STAMPEDE trialists advocate that upfront docetaxel is considered for metastatic hormone-naïve prostate cancer patients regardless of metastatic burden.
Regarding treatment selection in men with mCRPC, the TOPARP-B study assessed patients with DDR gene aberrations who were randomly assigned (1:1) to receive 400 mg or 300 mg olaparib twice daily, given continuously in 4-week cycles until disease progression or unacceptable toxicity.4 The primary endpoint of confirmed response was defined as a composite of all patients presenting with any of the following outcomes: radiological objective response, a decrease in PSA of 50% or more (PSA50) from baseline, or conversion of circulating tumor cell count (from ≥5 cells per 7.5 mL blood at baseline to <5 cells per 7.5 mL blood). Among 711 patients, 161 patients had DDR gene aberrations, 98 of whom were randomly assigned and treated (49 patients for each olaparib dose), with 92 evaluable for the primary endpoint (46 patients for each olaparib dose). Median follow-up was 24.8 months (IQR 16.7-35.9). The confirmed composite response was achieved in 25 (54.3%; 95% CI 39.0-69.1) of 46 evaluable patients in the 400 mg cohort, and 18 (39.1%; 25.1-54.6) of 46 evaluable patients in the 300 mg cohort. Radiological response was achieved in eight (24.2%; 11.1-42.3) of 33 evaluable patients in the 400 mg cohort and six (16.2%; 6.2-32.0) of 37 in the 300 mg cohort. A PSA50 response was achieved in 17 (37.0%; 23.2-52.5) of 46 and 13 (30.2%; 17.2-46.1) of 43 patients. A circulating tumor cell count conversion was achieved in 15 (53.6%; 33.9-72.5) of 28 and 13 (48.1%; 28.7-68.1) of 27 patients. In this cohort, BRCA1/2 and PALB2 had the highest response rates, whereas there were few responses for ATM and CDK12 patients.
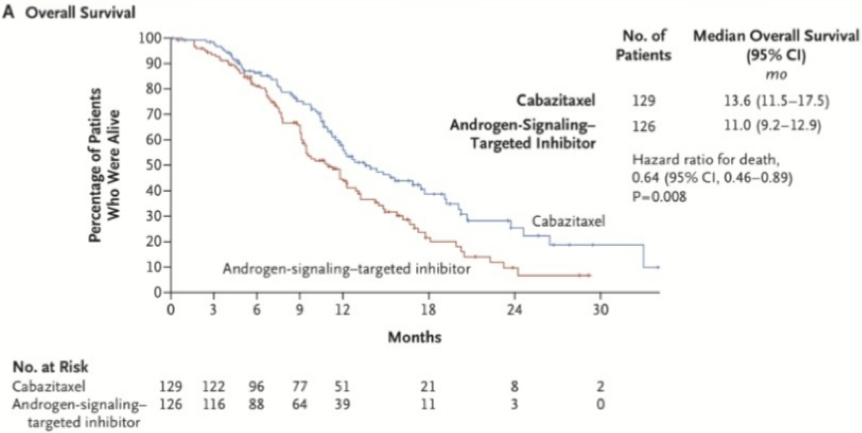
The recently published CARD trial was also one of Dr. Morgans’ most impactful studies of the past year.5 This trial randomly assigned patients 1:1 who had previously received docetaxel and an androgen-signaling-targeted inhibitor (abiraterone or enzalutamide) to receive cabazitaxel or the other androgen-signaling-targeted inhibitor (either 1000 mg of abiraterone plus prednisone daily or 160 mg of enzalutamide daily). The primary endpoint was imaging-based PFS. There were 255 patients that underwent randomization. After a median follow-up of 9.2 months, imaging-based progression or death was reported in 95 of 129 patients (73.6%) in the cabazitaxel group, as compared with 101 of 126 patients (80.2%) in the group that received an androgen-signaling-targeted inhibitor (HR 0.54; 95% CI 0.40-0.73; p <0.001). The median imaging-based progression-free survival was 8.0 months with cabazitaxel and 3.7 months with the androgen-signaling-targeted inhibitor. The median overall survival was 13.6 months with cabazitaxel and 11.0 months with the androgen-signaling-targeted inhibitor (HR 0.64, 95% CI 0.46-0.89; p = 0.008).
Dr. Morgans notes that in general sequencing AR agents is poorly effective: 14% of patients had a >=50% PSA response to second AR targeted agent and median PFS was 2.7 month for the second AR agent.
Dr. Morgans concluded her presentation with several summary points:
- The treatment landscape for mCSPC is evolving – there are now four systemic agents with similar effectiveness available. Shared decision making, including patient preferences, can be useful
- There is new data to support making treatment decisions in mCRPC – this includes personalization of treatment with PARP inhibitors coming soon, the first third-line study in mCRPC demonstrating OS benefit with cabazitaxel, and highlighting that sequencing of AR targeted agents is likely ineffective and delays time to effective systemic therapy
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Davis, Ian D., Andrew J. Martin, Martin R. Stockler, Stephen Begbie, Kim N. Chi, Simon Chowdhury, Xanthi Coskinas et al. "Enzalutamide with standard first-line therapy in metastatic prostate cancer." New England Journal of Medicine 381, no. 2 (2019): 121-131.
2. Chi, Kim N., Neeraj Agarwal, Anders Bjartell, Byung Ha Chung, Andrea J. Pereira de Santana Gomes, Robert Given, Álvaro Juárez Soto et al. "Apalutamide for metastatic, castration-sensitive prostate cancer." New England Journal of Medicine 381, no. 1 (2019): 13-24.
3. Clarke, N. W., A. Ali, F. C. Ingleby, A. Hoyle, C. L. Amos, G. Attard, C. D. Brawley et al. "Addition of docetaxel to hormonal therapy in low-and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial." Annals of Oncology 30, no. 12 (2019): 1992-2003.
4. Mateo, Joaquin, Nuria Porta, Diletta Bianchini, Ursula McGovern, Tony Elliott, Robert Jones, Isabel Syndikus et al. "Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial." The Lancet Oncology 21, no. 1 (2020): 162-174.
5. de Wit, Ronald, Johann de Bono, Cora N. Sternberg, Karim Fizazi, Bertrand Tombal, Christian Wülfing, Gero Kramer et al. "Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer." New England Journal of Medicine 381, no. 26 (2019): 2506-2518.


