There has been almost no progress at all during the last 30 years in the management of advanced urothelial carcinoma (figure 1). However, in the last 5 years, five new immunotherapeutic agents have been introduced for the treatment of advanced urothelial carcinoma (figure 2), bringing significant improvement for advanced urothelial bladder cancer patients.
Figure 1 – 30 years of almost no progress in the treatment of urothelial carcinoma until very recently:
Figure 2 – Introduction of 5 new systemic agents in recent years:
Bladder cancer has a high somatic mutation rate, making it a good candidate for the development of new immunotherapeutic drugs. Additionally, a new classification of urothelial cancer by molecular markers has been introduced in recent years (Figure 3), with some showing preference for immunotherapeutic drugs.
Figure 3 – New classification or urothelial cancer by molecular markers:
Dr. Sternberg continued to discuss the role of immune-checkpoint inhibitors in the various treatment settings. In the platinum-refractory setting the immune checkpoint inhibitors have shown promising results (Table 1) with objective response rates ranging between 13.4-21.1%, showing improvement of progression-free survival and overall survival.
When assessing atezolizumab in the platinum-treated metastatic urothelial carcinoma patients in the Imvigor 2010 trial, the overall survival was shown to be affected by the PD-L1 status (figure 4).
Figure 4- IMvigor 210 – overall survival with atezolizumab in the platinum treated metastatic urothelial carcinoma patients
Interestingly, alterations in DNA damage response and repair genes were associated with PD1/PDL1 blockade response and survival1.
The Keynote-045 phase 3 pembrolizumab study in platinum-refractory patients was an international randomized, open label Phase III study comparing pembrolizumab to chemotherapy. The primary endpoint was overall survival and progression-free survival. The treatment was continued for 2 years2. The results demonstrated a 27% reduction in the risk of death with pembrolizumab (Figure 5).
Figure 5 – Pembrolizumab in platinum-refractory patients:
Atezolizumab also demonstrated similar beneficial results when compared to chemotherapy in the same setting, with an improvement in overall survival3 (Figure 6).
Figure 6 – Atezolizumab Phase III vs. chemotherapy overall survival:
Immune checkpoint inhibitors have also been assessed as first-line in cisplatin-ineligible patients. Both pembrolizumab and atezolizumab have shown an objective response rate of 29% and 23%, respectively, with improvement in median overall survival (Figure 7).
Figure 7 – Immune checkpoint inhibitors as first-line in cisplatin-ineligible patients
The updated overall survival results of the Keynote-052 study, assessing pembrolizumab in cisplatin-ineligible patients demonstrated a significant improvement in 2-year overall survival in patients with a CPS>10 (47% vs. 24%).
An excellent summary slide of key first-line Phase III trials of anti-PD-1/PD-L1 antibodies in urothelial cancer is shown in figure 8.
Figure 8 – Summary of key first-line Phase III trials of immune checkpoint inhibitors
Dr. Sternberg moved on to discuss the results of the IMvigor 130 study (Figure 9) assessing and comparing atezolizumab monotherapy to combination with chemotherapy or chemotherapy alone. The intention to treat analysis results of the outcome of progression-free survival and overall survival are shown in Figure 10, and figure 11, respectively, demonstrating an advantage for the atezolizumab + chemotherapy combination arm.
Figure 9 - IMvigor 130 trial design:
Figure 10 – Imvigor 130 progression-free survival intention to treat analysis results:
Figure 11 – Imvigor 130 overall survival intention to treat analysis results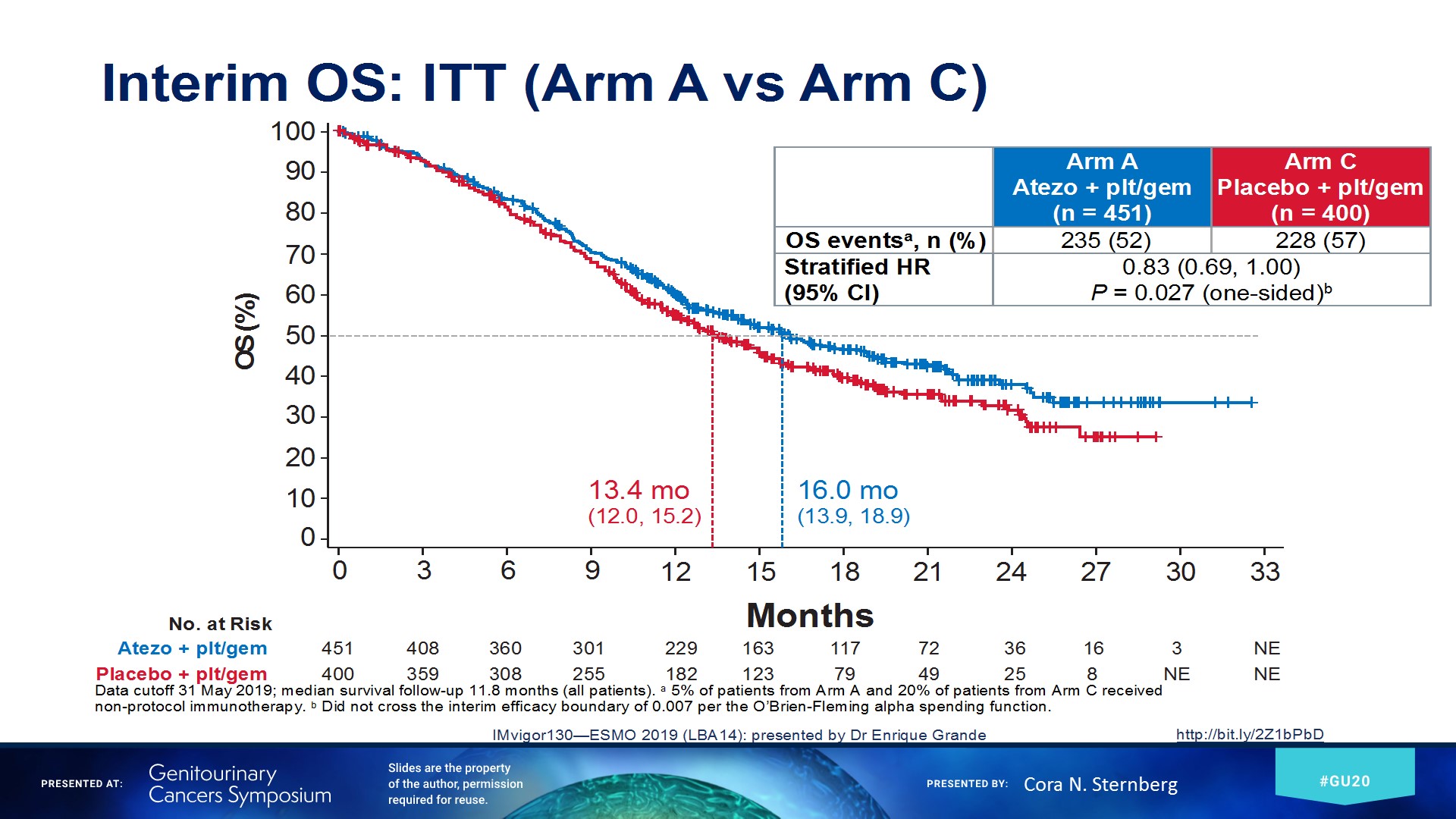
The JAVELIN Bladder 100 trial (NCT02603432) was discussed next. This study assessed patients with locally advanced or metastatic urothelial carcinoma following 1st line chemotherapy, who have not progressed and randomized to either standard of care or avelumab (Figure 12). On January 6, 2020, this trial met its primary endpoint of overall survival at the planned interim analysis.
Figure 12 – JAVELIN bladder cancer trial design:
New approaches in metastatic urothelial carcinoma have been developed in the meantime. These include the antibody-drug conjugates. These antibodies are conjugated to a cytotoxic drug or radionuclide. They have been shown to improve the potency and effectiveness of these antibodies. This type of therapy enables targeted delivery of the toxic payload to tumor cells, minimizing non-specific, systemic toxicity. An excellent example is enfortumab vedotin (EV). It targets Nectin-4, highly expressed in cancer cells. In the study assessing its effectiveness in patients with locally advanced or metastatic disease who have been treated previously with cisplatin and immune checkpoint inhibitors, the results demonstrated an objective response rate of 44%. There was a 12% and 32% complete and partial response rate, respectively. There have been other antibodies that have been studied, including sacituzumab govetecan.
Dr. Sternberg concluded her excellent talk pointing out a few important factors. The high mutational complexity that exists in bladder urothelial cancer, has the potential for many neoantigens, possibly triggering an immune response. Immunotherapy is currently the first-line recommended therapy for 1st line cisplatin-ineligible patients and second-line after cisplatin-based chemotherapy. The currently studied combinations in the first line including chemotherapy and immunotherapy are of great interest. Lastly, new therapeutic approaches including antibody-drug conjugates are of great interest.
Presented by: Cora Sternberg MD, FACP Clinical Director of the Israel Englander Institute for Precision Medicine, Weill Cornell Medicine
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New York, Twitter: @GoldbergHanan at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Teo, Min Yuen, Kenneth Seier, Irina Ostrovnaya, Ashley M. Regazzi, Brooke E. Kania, Meredith M. Moran, Catharine K. Cipolla et al. "Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers." Journal of Clinical Oncology 36, no. 17 (2018): 1685.
2. Bellmunt, Joaquim, Ronald De Wit, David J. Vaughn, Yves Fradet, Jae-Lyun Lee, Lawrence Fong, Nicholas J. Vogelzang et al. "Pembrolizumab as second-line therapy for advanced urothelial carcinoma." New England Journal of Medicine 376, no. 11 (2017): 1015-1026.
3. Powles, Thomas, Ignacio Durán, Michiel S. Van Der Heijden, Yohann Loriot, Nicholas J. Vogelzang, Ugo De Giorgi, Stéphane Oudard et al. "Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial." The Lancet 391, no. 10122 (2018): 748-757.


