San Francisco, CA (UroToday.com) Sipluleucel-T (SIP-T) is an FDA-approved autologous cellular immunotherapy vaccine for metastatic castrate-resistant prostate cancer (mCRPC), manufactured by activating peripheral blood mononuclear sales collected by apheresis and culturing them with PA2024 (PAP fused to human GC-CSF) (Figure 1).
Figure 1:
Overall survival (OS) in metastatic castration resistant prostate cancer (mCRPC) is positively correlated with key product parameters of SIP-T. These include cumulative CD54 upregulation, CD54+ cell count; and total nucleated cell (TNC) count.
These product parameters were amplified in men with earlier stage prostate cancer versus metastatic castrate resistant prostate cancer, including increased T cell trafficking to the prostate and greater CD54 upregulation and larger immune responses.
The PROVENT trial (NCT03103152) aims to evaluate SIP-T treatment in men with Gleason grade 1 or 2 prostate cancer receiving active surveillance. The trial design is shown in Figure 2 and basic demographic details are shown in Table 1.
Fig. 2 ProVent study schema
Table 1. Baseline Patient Characteristics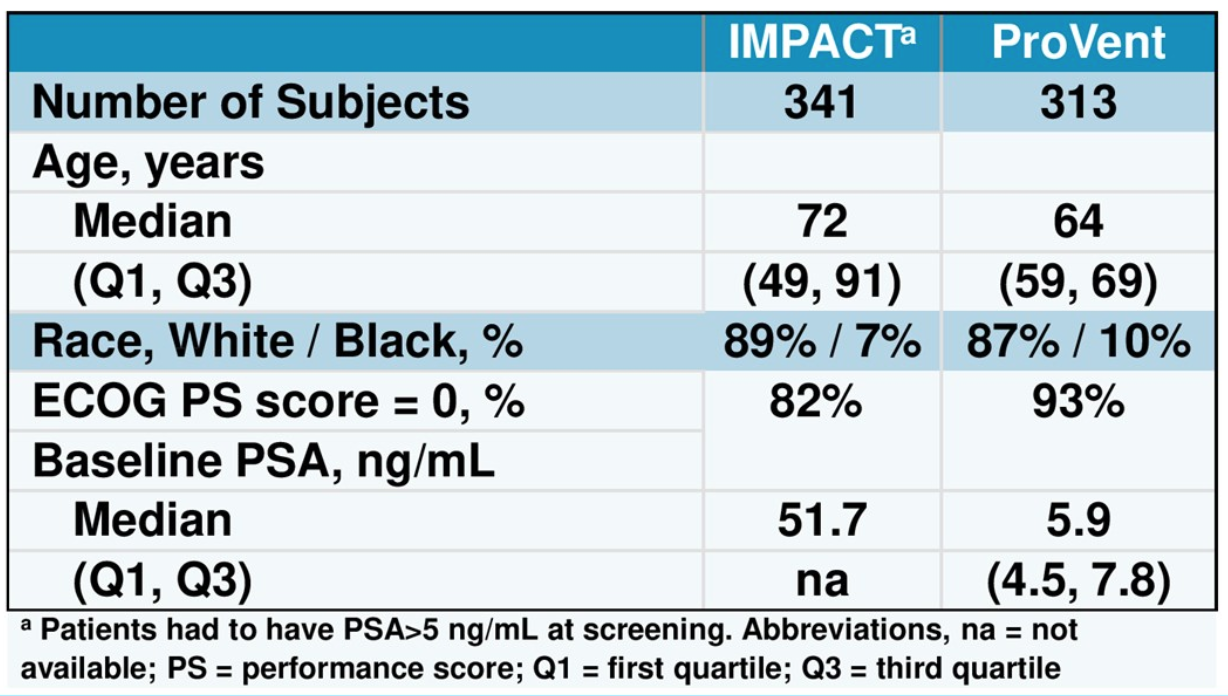
The authors of this poster compared product parameter outcomes in men in the phase 3 PROVENT trial with those from men with mCRPC in the phase 3 randomised IMPACT trial (NCT00065442).
Key final product parameters including CD54 upregulation, CD54+ cell count, and TNC count were analysed and compared.
The PROVENT apheresis cell counts, final product TNC, CD54+ cell counts, and CD54 upregulation are shown in Figure 4, 5 and 6, respectively.

Fig. 4 ProVent Apheresis Cell Counts
Fig. 5 ProVent Final Product TNC and CD54+ Cell Counts
Fig. 6 ProVent CD54 Upregulation
Based on the results showed in the figures, the authors concluded that men in the PROVENT trial tended to be younger and have better performance status and have lesser burden of disease than men in the IMPACT study, as could be expected. Apheresis yielded more TNC and CD54+ cells in men in PROVENT compared to IMPACT. Furthermore, final product CD54+ cell counts, CD54 upregulation and final product TNC were statistically higher in men enrolled in PROVENT than in IMPACT.
These findings suggest PROVENT subjects have a healthier immune system than those in IMPACT, resulting in the production and delivery of higher and more active doses in PROVENT than in IMPACT.
Presented by: Ashley Ross, MD, PhD, Mary Crowley Cancer Research, Dallas, TX, USA
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California.


