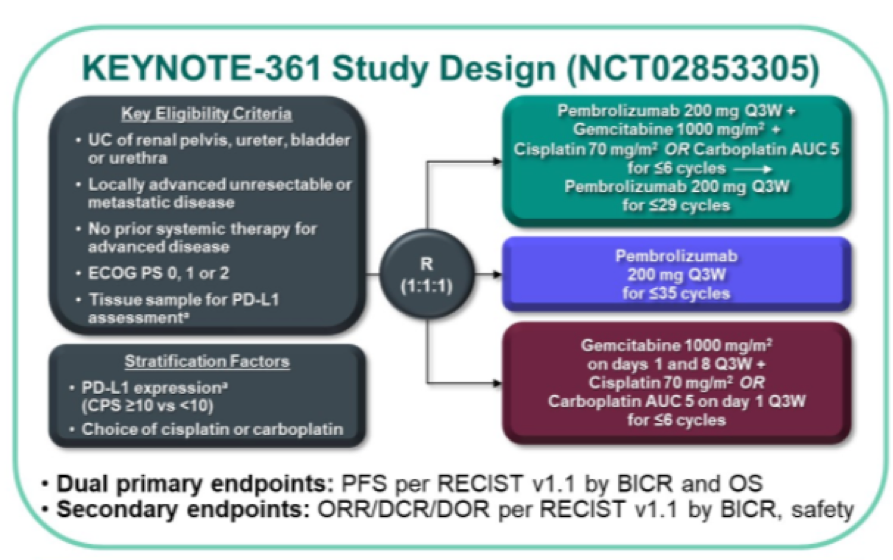
Unfortunately, as previously reported, the trial did not meet its primary endpoints, failing to demonstrate a benefit of pembrolizumab + chemotherapy vs chemotherapy alone based on progression-free or overall survival though formal testing of overall survival for pembrolizumab vs chemotherapy was not performed. In a poster presentation at the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Dr. Loriot presented a post hoc landmark analysis to examine the durability of complete responses (CR), partial responses (PR), and stable disease (SD), as well as long-term survival among patients who achieved CR, PR, or SD at week 9 of the KEYNOTE-361 trial.
Utilizing the intention to treat population of the KEYNOTE-361 trial, the authors included patients who had a best response of CR/PR/SD per RECIST v1.1 by blinded independent central review at the landmark date of week 9 (first imaging assessment per study protocol). The authors then assessed the duration of these responses and assessed overall survival (in a landmark analysis based on the 9-week assessment) using the Kaplan Meier method.
The authors identified 307 patients randomized to pembrolizumab and 352 randomized to chemotherapy, with median follow-up of 32.5 months and 31.4 months in these groups, respectively.
At 9-week assessment, patients who received pembrolizumab were less likely to have CR/PR/SD (n=137 [45%]) than those who received chemotherapy (n=253 [72%]). However, the duration of response was longer among patients who received pembrolizumab: 18.7 months vs 12.3 months for those who achieved a complete response and 35.0 vs 6.1 months for those who achieved PR. Duration of stable disease was 4.8 months and 4.6 months, respectively for patients who received pembrolizumab and chemotherapy.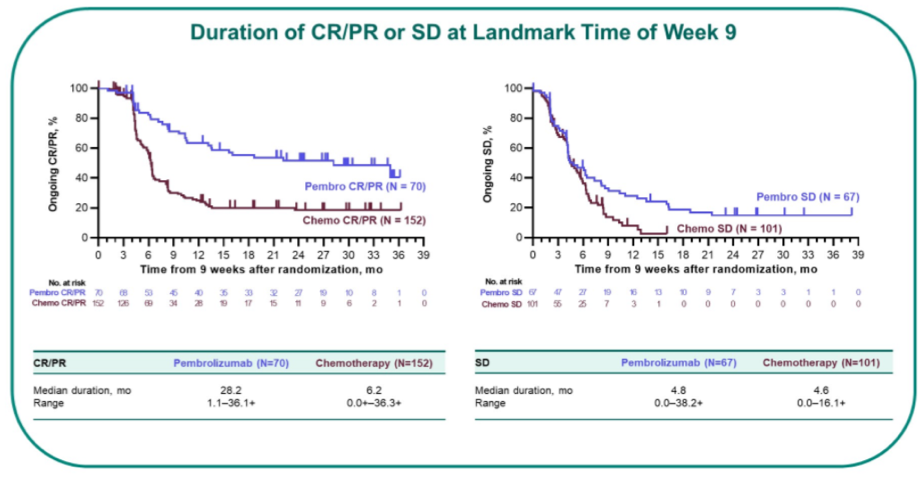
Median overall survival was not reached in either group for patients who achieved CR; was not reached (pembrolizumab) vs 15 months (chemotherapy) for patients who achieved PR, and was 19 months (pembrolizumab) vs 11 months (chemotherapy) for patients who had stable disease.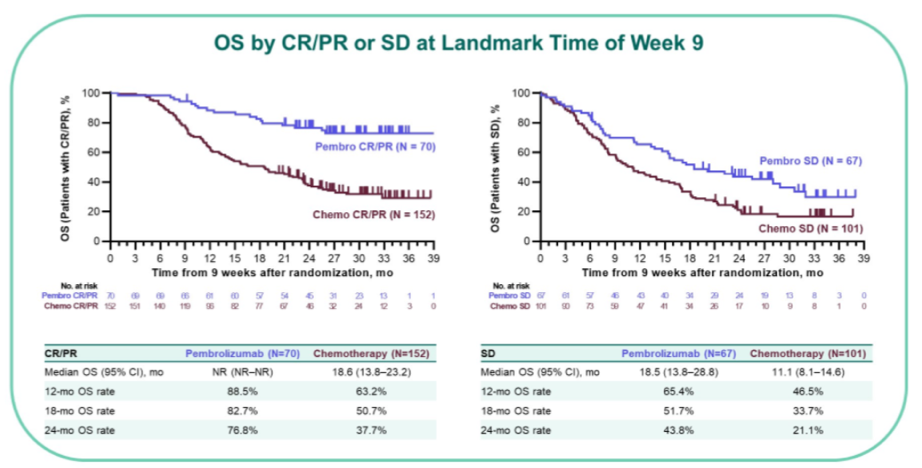
Across each response group (CR vs PR vs SD), pembrolizumab had numerically higher survival rates from 6 to 24 months.
The authors conclude that, while patients who received chemotherapy were more likely to have initial responses at 9-weeks, among those who responded, these were better sustained in those patients who received pembrolizumab.
Presented by: Yohann Loriot, MD, PhD, Institut de Cancérologie Gustave Roussy, Villejuif
Co-Authors: Ajjai Shivaram Alva, Tibor Csőszi, Mustafa Ozguroglu, Nobuaki Matsubara, Lajos Geczi, Susanna Y. Cheng, Yves Fradet, Stephane Oudard, Christof Vulsteke, Rafael Morales-Barrera, Aude Flechon, Seyda Gunduz, Alejo Rodriguez-Vida, Ronac Mamtani, Evan Y. Yu, Chih-Chin Liu, Kentaro Imai, Blanca Homet Moreno, Thomas Powles
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


