(UroToday.com) Most patients newly diagnosed with bladder cancer have non-muscle invasive disease (NMIBC). For patients with intermediate or high-risk NMIBC and those with carcinoma in situ (CIS), adjuvant treatment with BCG is guideline-recommended on the basis of proven benefits in disease recurrence. While BCG is efficacious, many patients eventually develop BCG-unresponsive disease. For many years, there have been very limited options for these patients. Radical cystectomy has remained the gold standard though numerous approaches including intravesical and systemic therapies have been investigated. Two such approaches are the use of systemic pembrolizumab (which is FDA approved) and salvage intravesical chemotherapy. In a plenary abstract presentation in the Rapid Abstract Session: Urothelial Carcinoma and Rare Tumors session at the 2021 ASCO GU Cancers Symposium, Dr. Vidit Sharma and colleagues presented a cost-effectiveness analysis comparing pembrolizumab with radical cystectomy and salvage intravesical chemotherapy (using gemcitabine-docetaxel as the prototypical regimen) for patients with BCG-unresponsive CIS.
To do so, the authors constructed a decision-analytic Markov model comparing pembrolizumab, salvage intravesical chemotherapy (with gemcitabine-docetaxel), and radical cystectomy for patients with BCG-unresponsive CIS +/- papillary tumors who are radical cystectomy candidates (index patient 1) or are unwilling/unable to undergo radical cystectomy (index patient 2). For each treatment approach, the authors considered a variety of outcomes following treatment including surveillance, recurrence, progression to MIBC, progression to metastasis, treatment toxicity, and death. They calculated incremental Cost-Effectiveness Ratios (ICERs) and compared these using a willingness-to-pay threshold of $100,000/Quality-adjusted life year (QALY). The model used a US Medicare perspective with a 5-year time horizon for the base case. Further, the authors performed a number of one-way and probabilistic sensitivity analyses for all model parameters.
In index patient 1, who was a candidate for radical cystectomy, pembrolizumab was not cost-effective vs. radical cystectomy (ICER $1,403,008) or salvage intravesical chemotherapy (ICER $2,011,923). On one-way sensitivity analysis, pembrolizumab only became cost-effective relative to radical cystectomy with a > 93% price reduction. Relative to radical cystectomy, salvage intravesical chemotherapy was cost-effective for time horizons < 5 years and nearly cost-effective at 5 years (ICER $118,324).
On one-way sensitivity analysis, salvage intravesical chemotherapy became cost-effective relative to radical cystectomy if its risk of recurrence or metastasis at 2 years was less than 55% or 5.9%, respectively.
For index patient 2 who was not a candidate for radical cystectomy, pembrolizumab required > 90% price reduction to be cost-effective vs. radical cystectomy (ICER $1,073,240). Probabilistic sensitivity analyses revealed that pembrolizumab was unlikely to be cost-effective even at high willingness-to-pay thresholds.
Further sensitivity analyses found that no two-way combination of extrapolated values resulted in pembrolizumab being favored over radical cystectomy or salvage intravesical chemotherapy for either index patient. Additionally, regardless of willingness to pay threshold, there was no situation in which pembrolizumab was cost-effective.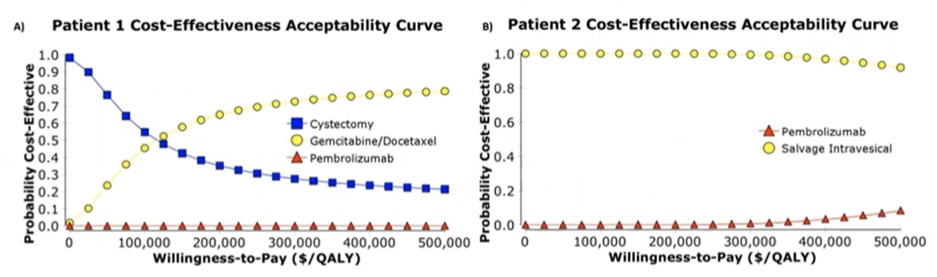
The authors conclude that, based on decision-analytic Markov modeling of treatment options for patients with BCG-unresponsive CIS, pembrolizumab was unlikely to be cost-effective without a > 90% price reduction.
Presented by: Vidit Sharma, MD, Mayo Clinic Rochester, MN
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


