The SGNTUC-019 basket study is evaluating tucatinib in combination with trastuzumab in patients with HER2+ or HER2-mutated solid tumors, including a cohort of patients with locally advanced or metastatic urothelial cancer. At the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Dr. Evan Yu and colleagues presented the study design for SGNTUC-019.
The mechanism of the tyrosine kinase inhibitor selective for HER2 tucatinib is as follows:

SGNTUC-019 (NCT04579380) is a multi-cohort, open-label, international phase II study evaluating patients with previously treated solid tumors displaying HER2 overexpression/amplification or activating mutations. Eligible patients must have HER2+ or HER2-mutated locally advanced or metastatic urothelial cancer solid tumors, with progression on or after the last systemic therapy for advanced disease. Patients must be ≥18 years old, with ECOG PS ≤1, adequate hepatic, hematological, renal, coagulatory, and cardiac function, and no prior exposure to HER2-directed therapy. For eligibility, HER2 alterations can be demonstrated by HER2 overexpression/amplification in tumor tissue by prior IHC/ISH (IHC 3+/signal ratio ≥2.0 or gene copy number >6), or by HER2 amplification/mutation in a prior or on-study next generation sequencing assay of ctDNA or prior tissue next-generation sequencing assay.
The HER2 overexpression/amplification urothelial cancer cohort will enroll 12 RECIST 1.1 response-evaluable patients. If more than two responses are observed, the cohort will be expanded to a total of 30 patients. Those with HER2-mutated urothelial cancer will be enrolled in a cohort of 30 patients for all solid tumor types except breast cancer and non-squamous NSCLC. If justified, a separate cohort for HER2-mutated urothelial cancer may be opened.
The study schema for SGNTUC-019 is as follows: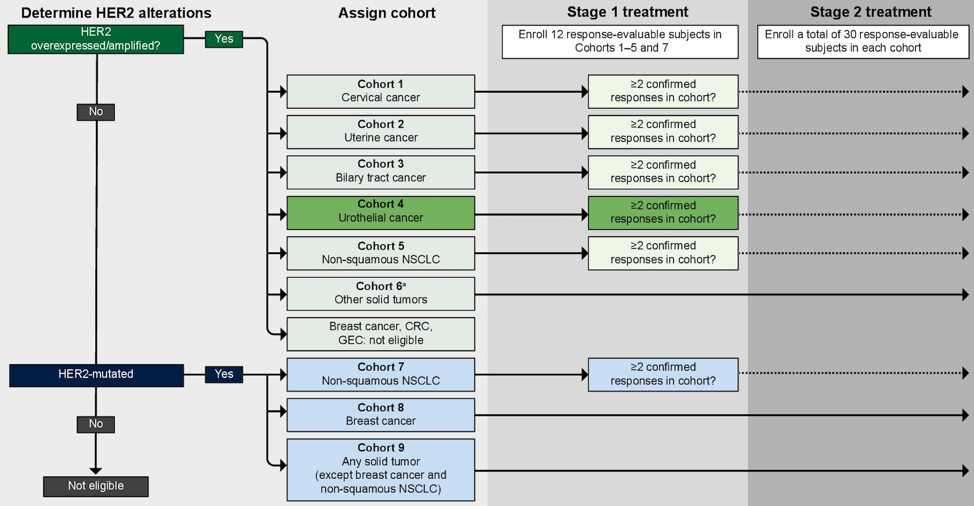
The primary objective for this trial is antitumor activity in each cohort, with confirmed objective response rate as the primary endpoint, and disease control rate, duration of response, progression free survival, and overall survival as secondary endpoints. Patients will receive tucatinib 300 mg orally twice daily and trastuzumab 8 mg/kg IV on cycle 1 day 1 and 6 mg/kg q21 days from cycle 2 day 1. Disease assessments per RECIST 1.1 will occur every six weeks for 24 weeks, then every 12 weeks. Trough concentrations of tucatinib will be evaluated in all patients in cycles 2-6, with a peak concentration sampled in cycle 3. Quality of life will be evaluated every two cycles using EQ-5D-5L. The study is open and enrolling with an estimated study end date of the first quarter in 2023. Approximately 75 sites are planned in North America, Asia-Pacific, and Europe.
Clinical trial information: NCT04579380
Presented by: Evan Y. Yu, Division of Oncology, Department of Medicine, University of Washington, Seattle, WA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021

