Enfortumab vedotin is an antibody-drug conjugate directed to Nectin-4, a cell adhesion molecule highly expressed in urothelial carcinoma. Phase I and II clinical trials previously demonstrated consistent clinical benefits, with durable clinical responses achieved and objective response rates of >40%.1-2 Enfortumab vedotin received accelerated approval from the US FDA in 2019. At the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU), Dr. Thomas Powles and colleagues presented results of EV-301, a phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated locally advanced or metastatic urothelial carcinoma.
EV-301 (NCT03474107) is a global, open-label phase III study of enfortumab vedotin versus chemotherapy conducted in patients with locally advanced or metastatic urothelial carcinoma who had received a prior platinum-containing chemotherapy and had disease progression during or after PD-1/L1 inhibitor treatment. Patients were randomized 1:1 to receive enfortumab vedotin (1.25 mg/kg) on Days 1, 8, and 15 of each 28-day cycle or investigator choice of standard docetaxel, paclitaxel, or vinflunine chemotherapy. The trial design for EV-301 was as follows:

The primary endpoint was overall survival, and secondary endpoints included investigator-assessed progression-free survival, objective response rate, and disease control rate per RECIST v1.1, as well as safety and tolerability. Enrollment of approximately 600 patients provided 85% power to detect a statistically significant difference at an overall 1-sided 0.025 type I error rate with a hazard ratio of 0.75, median overall survival of 8 months for chemotherapy, and dropout rate of 10%. A prespecified interim analysis, which tested overall survival at an adjusted 1-sided significance level of p = 0.00679, was performed when ≥285 deaths had occurred.
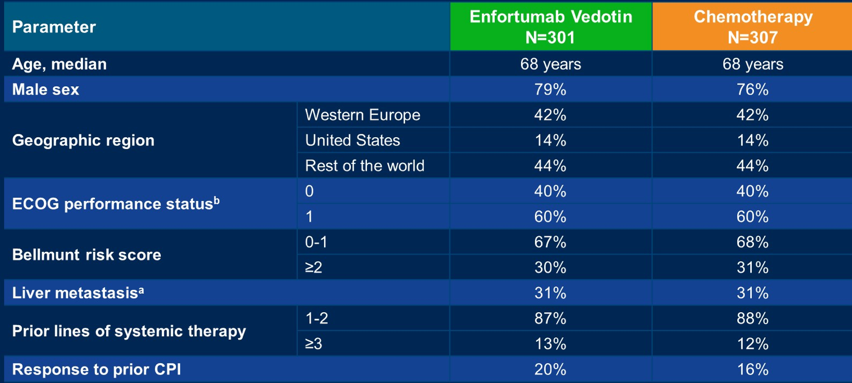
Overall, 608 patients with locally advanced or metastatic urothelial carcinoma were randomly assigned to enfortumab vedotin (n=301) or chemotherapy (n=307). Baseline characteristics of the trial population are as follows:
As of July 15, 2020, 301 deaths had occurred (enfortumab vedotin, n=134; chemotherapy, n=167). The median time on treatment for patients receiving enfortumab vedotin was 5.0 months (range: 0.5-19.4) and was 3.5 months for chemotherapy (range: 0.2-15.0). Treatment discontinuation occurred in 81% of patients receiving enfortumab vedotin, compared to 93% of patients receiving chemotherapy, most commonly secondary to progressive disease (59% for both arms). After an 11.1 month follow-up, median overall survival was significantly prolonged by 3.9 months with enfortumab vedotin compared with chemotherapy (median overall survival: 12.9 vs 9.0 months, respectively; HR 0.70, 95% CI 0.56-0.89, 1-sided p = 0.00142):
Additionally, the overall survival benefit of enfortumab vedotin was retained in the majority of prespecified subgroups:
Progression-free survival also was improved with enfortumab vedotin (5.6 months) versus chemotherapy (3.7 months) (HR 0.62, 95% CI 0.51-0.75; 1-sided p<0.00001):

Both objective response rate (40.6% versus 17.9%, 1-sided p<0.001) and disease control rate (71.9% versus 53.4%, 1-sided p<0.001) were significantly higher with enfortumab vedotin versus chemotherapy. Rates of treatment-related adverse events (93.9% vs 91.8%), including serious treatment-related adverse events (22.6% vs 23.4%), were comparable between the enfortumab vedotin and chemotherapy groups. Rates of grade ≥3 treatment-related adverse events were ~50% in both groups, with decreased neutrophil count (13.4%) and white blood cell count (6.9%) more common in the chemotherapy group, and maculo-papular rash (7.4%) more common in the enfortumab vedotin group.
Dr. Powles concluded his presentation of the EV-301 phase 3 randomized controlled trial with the following summary remarks:
- Enfortumab vedotin had superior overall survival compared with chemotherapy in patients with advanced urothelial carcinoma who had previously received platinum-based chemotherapy and a PD-1/L1 inhibitor. Enfortumab vedotin showed superior progression-free survival and response rates compared with chemotherapy, subgroup analyses also broadly showed benefit in the enfortumab vedotin arm, and results were consistent with phase 1 and 2 studies
- Enfortumab vedotin demonstrated a tolerable and manageable safety profile, with no new safety signals identified. Adverse events of special interest were generally mild/moderate in severity and consistent with those reported in prior studies
- Enfortumab vedotin is the first drug, beyond chemotherapy and immunotherapy, to show significant survival advantage in previously treated advanced urothelial carcinoma
Following Dr. Powles presentation, EV-301 was published in the New England Journal of Medicine.3
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
References:
- Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: A Phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol2020 Apr 1;38(10):1041-1049.
- Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of Enfortumab Vedotin in Urothelial Carcinoma after Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy.J Clin Oncol. 2019 Oct 10;37(29):2592-2600.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med2021 Feb 12 [Epub ahead of print].
Related Content:
Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma


