The first topic, growth rates during active surveillance for T1aN0M0 renal cell carcinoma (RCC), included two papers, the first of which was by Finelli et al. “Small Renal Mass Surveillance: Histology-specific Growth Rates in a Biopsy-characterized Cohort” published in European Urology.1 This study compared the growth rates and progression of different histologic subtypes of RCC small renal masses in a large cohort of patients with biopsy-characterized small renal masses on active surveillance. There were 136 biopsy-proven RCC small renal mass lesions managed by active surveillance, with treatment deferred until progression or patient/surgeon decision. The median follow-up for patients who remained on active surveillance was 5.8 years (IQR 3.4-7.5). Finelli et al. noted that clear cell RCC small renal masses grew faster than papillary type 1 small renal masses (0.25 and 0.02 cm/yr on average, respectively, p = 0.0003):

As follows is the growth rate by histology, noting that 15% of clear cell RCC small renal masses grew >0.5 cm/year:

Overall, 60 RCC small renal mass lesions progressed: 49 (82%) by rapid growth (volume doubling), seven (12%) increasing to ≥4 cm, and four (6.7%) by both criteria. Six patients developed metastases, and all were of clear cell RCC histology.
The second study assessing growth rates during active surveillance for T1aN0M0 RCC was by Ball et al. entitled “Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention” published in the Journal of Clinical Oncology.2 The goal of this study was to evaluate the growth of genetically defined renal tumors and their association with patient clinical and genetic characteristics.
In this cohort from the NCI, there were 292 patients with 435 genetically defined tumors were identified, including 286 VHL-deficient, 91 FLCN-deficient, 52 MET-activated, and 6 BAP1-deficient tumors. The median follow-up was 3.6 years (IQR 2.0-5.6 years) and the median tumor growth rate for the entire cohort was 0.31 cm per year. Notably, BAP1-deficient tumors had the fastest median growth rate (0.6 cm/y, IQR 0.57-0.68 cm/y), followed by VHL-deficient tumors (growth rate 0.37 cm/y, IQR 0.25-0.57 cm/y), FLCN-deficient tumors (growth rate 0.10 cm/y, IQR 0.04-0.24 cm/y), and tumors with MET activation (growth rate 0.15 cm/y, IQR 0.053-0.32 cm/y; p < 0.001). When stratifying all tumors by histologic subtype, clear cell renal cell carcinoma had a significant growth rate of 0.37 cm/year, followed by papillary type 1 at 0.15 cm/year, chromophobe at 0.15 cm/year, and hybrid oncocytic tumors at 0.11 cm/year. Specifically related to VHL patients, young patients had faster-growing tumors: 0.40 cm/year vs 0.34 cm/year. As follows is a layered pie-chart of histology and genetic alteration:

Dr. Bhindi’s takeaway messages from these two studies are as follows:
- We are moving towards personalized care during activity surveillance as tumor growth rate depends on tumor histology. We should consider biopsy when it will change management
- Challenges for the future include a lack of data for young patients and those with high-grade tumors, as well as tumor growth rate being not always indicative of metastatic potential
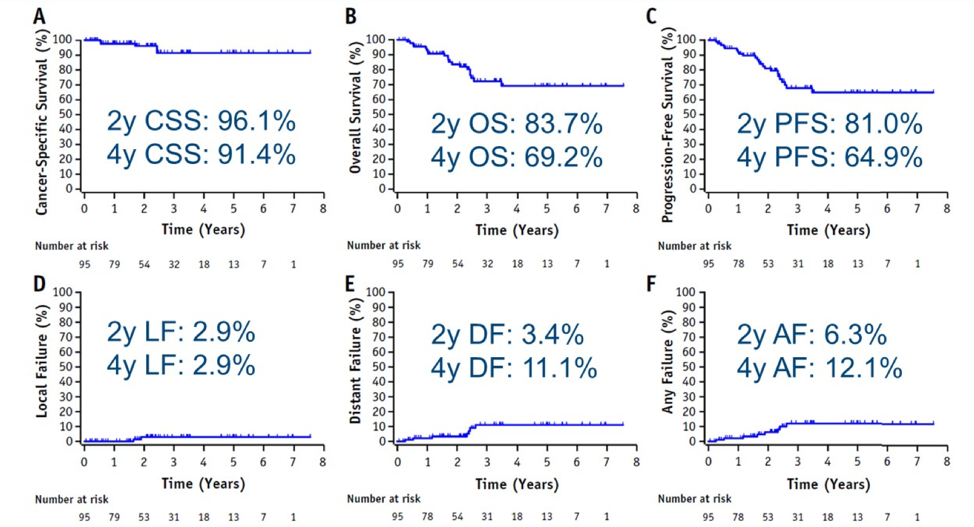
Dr. Bhindi’s takeaway messages from this study are as follows:
- Stereotactic ablative radiotherapy may be an emerging treatment option for inoperative T1b, M0 RCC with a low local failure rate with early follow-up and relatively well tolerated. However, we need more long-term data and data on T2 tumors
- Is there a role for “cytoreductive” stereotactic ablative radiotherapy in mRCC? The CYTOSHRINK trial is currently accruing and the SAMURAI trial is under development

Finally, Dr. Bhindi discussed “Deferred Cytoreductive Nephrectomy in Patients with Newly Diagnosed Metastatic Renal Cell Carcinoma” by Bhindi et al. published in European Urology.5 This study characterized outcomes of deferred cytoreductive nephrectomy after upfront sunitinib, outcomes relative to sunitinib alone, and outcomes of cytoreductive nephrectomy followed by sunitinib among patients with mRCC in the IMDC database. Among 1,541 patients, there were 651 (42%) that received sunitinib alone, 805 (52%) underwent cytoreductive nephrectomy followed by sunitinib, and 85 (5.5%) received sunitinib followed by deferred cytoreductive nephrectomy, at a median of 7.8 months from diagnosis. Median overall survival periods for patients treated with sunitinib alone was 10 months, cytoreductive nephrectomy followed by sunitinib was 19 months, and sunitinib followed by deferred cytoreductive nephrectomy was 46 months, while the median time to sunitinib treatment failure values were 4, 8, and 13 months, respectively. In multivariable regression analyses, sunitinib followed by deferred cytoreductive nephrectomy was significantly associated with improved overall survival (HR 0.45, 95% CI 0.33-0.60) and time to treatment failure (HR 0.62, 95% CI 0.46-0.85) versus sunitinib alone:

The next generation of cytoreduction trials are as follows:

Dr. Bhindi’s take-home points for these two papers are as follows:
- Deferred cytoreductive nephrectomy is supported in retrospective studies, however, it is awaiting evaluation in prospective phase III trials
- In the meantime, we should consider discussing these patients at multidisciplinary rounds
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
References:
- Finelli A, Cheung DC, Al-Matar A, et al. Small Renal Mass Surveillance: Histology-specific Growth Rates in a Biopsy-characterized Cohort. Eur Urol 2020 Sep; 78(3):460-467.
- Ball MW, An JY, Gomella PT, et al. Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention. J Clin Oncol. 2020 Apr 10;38(11):1146-1153.
- Siva S, Correa RJM, Warner A, et al. Stereotactic Ablative Radiotherapy for >=T1b Primary Renal Cell Carcinoma: A Report From the International Radiosurgery Oncology Consortium for Kidney (IROCK). Int J Radiat Oncol Biol Phys. 2020 Nov 15;108(4):941-949.
- de Bruijn R, Wimalasingham A, Szabados B, et al. Deferred Cytoreductive Nephrectomy Following Presurgical Vascular Endothelial Growth Factor Receptor-targeted Therapy in Patients with Primary Metastatic Clear Cell Renal Cell Carcinoma: A Pooled Analysis of Prospective Trial Data. Eur Urol Oncol. 2020 Apr;3(2):168-173.
- Bhindi B, Graham J, Wells JC, et al. Deferred Cytoreductive Nephrectomy in Patients with Newly Diagnosed Metastatic Renal Cell Carcinoma. Eur Urol 2020 Oct;78(4):615-623.
Small Renal Mass Surveillance: Histology-Specific Growth Rates in a Biopsy-Characterized Cohort - Beyond the Abstract


