The methodology of the CheckMate214 trial has been previously reported and published. In short, this trial randomized 1096 patients with clear cell advanced renal cell carcinoma to the combination immunotherapy approach of nivolumab plus ipilimumab (550 patients) or sunitinib (546 patients). The majority of patients had intermediate or poor risk disease (n=847). Patients in the immunotherapy arm received nivolumab plus ipilimumab every 3 weeks for 4 cycles followed by nivolumab monotherapy maintenance every 2 weeks while those in the sunitinib arm received daily oral therapy for cycles with 4-weeks on/2-weeks off. Patterns of progression were characterized in ITT pts and analyzed in a post hoc fashion using descriptive statistics. Progression patterns were defined by ≥ 20% target lesion growth (T), unequivocal progression of non-target lesions (NT), and new lesion(s) (NL). Response and progression were assessed per independent radiology review committee via RECIST v1.1.

The authors documented evidence of radiographic progression (RP) in 299 of 550 (54.4%) patients in the ITT population who received nivolumab plus ipilimumab as compared to 289 of 546 (52.9%) patients who were treated with sunitinib.
In the ITT population, confirmed responses were noted in 215 (39.1%) of patients treated with nivolumab plus ipilimumab and 177 (32.4%) of patients treated with sunitinib. Among these, 71 of 215 (33.0%) of those treated with nivolumab plus ipilimumab and 84 of 177 (47.5%) of those treated with sunitinib experience post-response RP. Further, 8 of 59 (13.6%) and 3 of 14 (21.4%) patients progressed after complete response after nivolumab plus ipilimumab versus sunitinib, respectively. Additionally, 63 of 156 (40.4%) and 81 of 163 (49.7%) patients progressed after partial response after nivolumab plus ipilimumab versus sunitinib, respectively.
Notably, patterns of RP were different between patients who received nivolumab plus ipilimumab and those who received sunitinib.
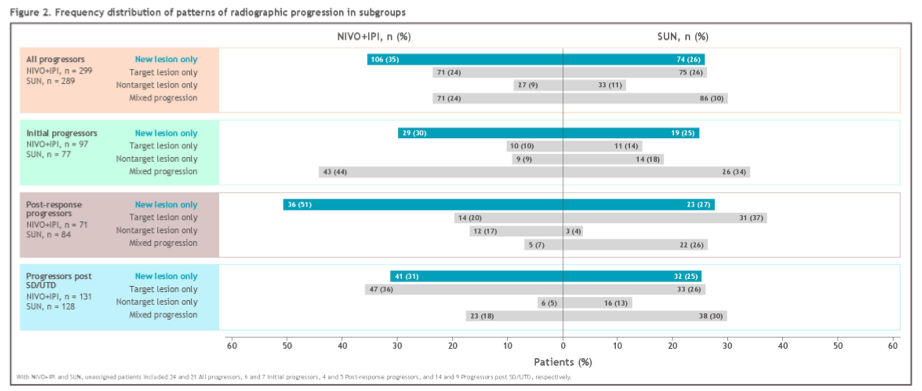
Among patients who received nivolumab plus ipilimumab, 106 of 299 (35.5%) RPs resulted from NL only compared to 74 of 289 (25.6%) of those who were treated with sunitinib, and this differential was more pronounced in patients with an initial confirmed response (36/71 [50.7%] vs 23/84 [27.4%]).
In contrast, most NL-only RPs in initial responders occurred in a single organ (34/36 [94.4%] for nivolumab plus ipilimumab; 20/23 [87.0%] for sunitinib) with the most common being lymph nodes (11/34 [32.4%]), brain (8/34 [23.5%]), and lung (5/34 [14.7%]) with nivolumab plus ipilimumab, and lymph nodes (7/20 [35.0%]), brain (4/20 [20.0%]) and adrenal gland (3/20 [15.0%]) with sunitinib.
The authors specifically noted that most lesions occurring in the brain were among patients with new lesions only.
The authors, therefore, conclude that there are different patterns of tumor relapse and disease progression with a long-term follow-up among patients treated with nivolumab plus ipilimumab and sunitinib in the CheckMate214. In particular new lesion-only progression was noted more often in patients receiving nivolumab plus ipilimumab, particularly among patients progressing following an initial response.
Presented by: Nizar M. Tannir, MD, FACP, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


