(UroToday.com) The treatment of germ cell tumors that relapse after or are refractory to platinum-based chemotherapy can include high dose chemotherapy (HDCT) with autologous stem-cell transplant (ASCT). Multiple cohorts from North America and Europe suggest that 5-year overall survival with HDCT ranges is between 48% and 65%. In this abstract, the authors conducted a retrospective and multi-center cohort study of outcomes from HDCT and ASCT in Australia and New Zealand between 1999 and 2019.
This study identified 111 patients who received treatment at 13 centers, with each center treating a median of 7 patients (range 1-27). The median follow-up available for patients was 4.4 years, and the characteristics of the patients are shown in the table below. More patients received 3 cycles of HDCT versus 1 or 2 cycles (44% versus 32% and 24%), and the most common regimen used was TI-CE (47%) followed by CE (31%). Five treatment-related deaths occurred, and 14% of patients experienced progressive disease on treatment.
Of the 103 patients assessed for outcomes, 56% of patients achieved a complete response to HDCT and ASCT. One, two, and five-year progression-free survival and overall survival rates are shown in the tables below. The overall survival at five years was comparable to other published cohorts.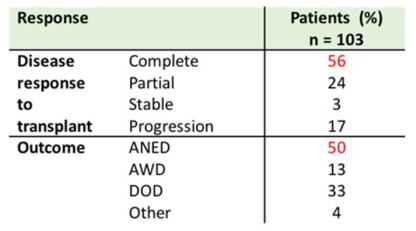

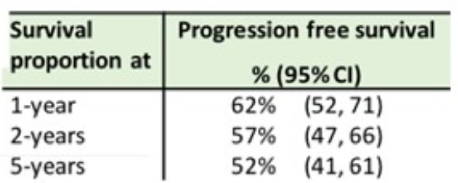
Outcomes were worse when patients were stratified by IGCCCG and IPFSG risk category, but only IPFSG risk category had a statistically significant association with overall survival. Toxicities noted included a 100% rate of grade 4 neutropenia and a 97% rate of grade 4 thrombocytopenia.
Presented by: Elizabeth Anne Connelly, MD, Stanford Medicine
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


