The investigators here presented their assessment of plasma samples collected from subjects in the Phase III PROfound study of olaparib versus physician’s choice of abiraterone or enzalutamide in genomically-qualified subjects with mCRPC. Matched plasma samples from subjects in Cohort A (tissue evidence of qualifying BRCA1, BRCA2, ATM alterations) were evaluated via the FoundationOne® Liquid CDx assay. Those with ctDNA evidence of such alterations (139/181 evaluable) were evaluated for radiographic progression-free survival (rPFS) via blinded independent central review. Of these, 111 (79.9%) had a qualifying alteration detected. Subjects receiving olaparib (n=73) had longer rPFS than those subjects receiving physician’s choice of abiraterone or enzalutamide (n-38). Via stratified log-rank test, the olaparib-treated subjects had a median rPFS of 7.39 months (95% confidence interval [CI] 5.65-10.38) versus 3.53 months (95% CI 1.77-3.71), for a hazard ratio [HR] of of 0.33 (0.21-0.53, two-sided p-value <0.0001). These data are consistent with the HR of 0.34 (0.25-0.47) in the intention-to-treat population of Cohort A from the tissue-based PROfound study. 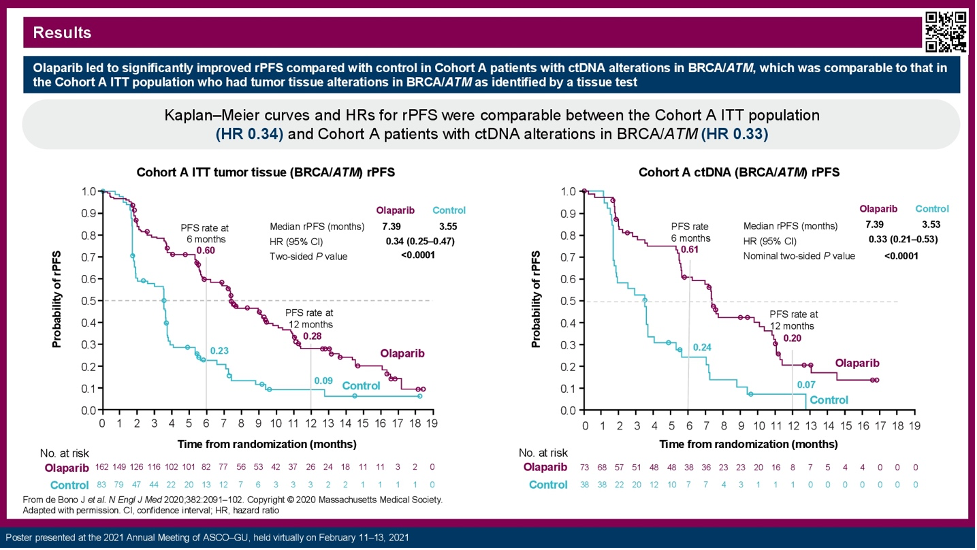
Although these data do not replace the use of tissue-based assays, they support the use of ctDNA for evaluation for BRCA1, BRCA2, and ATM alterations in men with mCRPC who are being considered for PARP inhibitor therapy, and with evidence for accurate prediction of response as compared to tissue testing.
Presented by: Nobuaki Matsubara, MD, Department of Breast and Medical Oncology, National Cancer Center Hospital East, Kashiwa, Chiba, Japan
Written by: Jones Nauseef, MD, PhD, Fellow, Division of Hematology and Oncology, Weill Cornell Medicine/New York Presbyterian Hospital, New York, New York, Twitter: @DrJonesNauseef during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021
References:
1. de Bono, Johann, Joaquin Mateo, Karim Fizazi, Fred Saad, Neal Shore, Shahneen Sandhu, Kim N. Chi et al. "Olaparib for metastatic castration-resistant prostate cancer." New England Journal of Medicine 382, no. 22 (2020): 2091-2102.
2. Abida, Wassim, Akash Patnaik, David Campbell, Jeremy Shapiro, Alan H. Bryce, Ray McDermott, Brieuc Sautois et al. "Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration." Journal of Clinical Oncology 38, no. 32 (2020): 3763.
3. Wyatt, Alexander W., Matti Annala, Rahul Aggarwal, Kevin Beja, Felix Feng, Jack Youngren, Adam Foye et al. "Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer." JNCI: Journal of the National Cancer Institute 109, no. 12 (2017).


