The schema of the study is shown below. In total, 982 patients were randomized in a 1:1 fashion to either the combination of apalutamide and abiraterone or abiraterone plus placebo. All patients were also on androgen deprivation therapy. The primary endpoint of the study was radiographic progression free survival. In this presentation, Dr. Dana Rathkopf presented final results from a data cutoff of September 2020.

Regarding patient characteristics, baseline clinical characteristics between the two study arms were similar, including comparable rates of bone as well as visceral liver and lung metastases.

The overall data from this trial are summarized below in the table. Notably, the trial met its primary endpoint of rPFS benefit with androgen annihilation, prolonging rPFS from 16.6 months to 22.6 months (HR 0.69, p < 0.0001). However, after 54.8 months of median follow-up at the September 2020 data cutoff, overall survival was numerically higher but not statistically significantly higher with androgen annihilation (36.2 months versus 33.7 months, p = 0.498).


Across all pre-specified subgroups, including the presence of visceral metastases and age >= 75 years, there was a trend towards benefit from combination androgen annihilation.

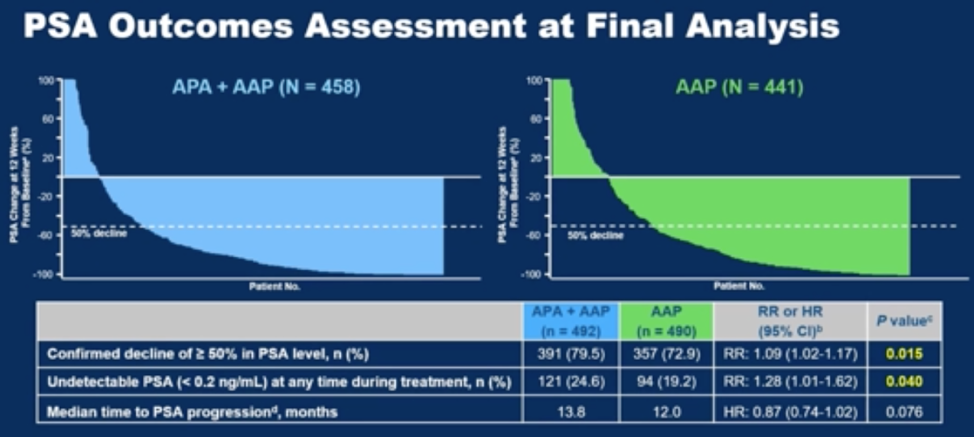
Other secondary and exploratory endpoints, including time to initiation of cytotoxic chemotherapy, pain progression, chronic opioid use, and second progression free survival were similar between groups. Two-third of patients on trial were able to go on and receive another life prolonging therapy (70% chemotherapy, ~20% another hormonal therapy). Finally, more patients experienced a decline in PSA of greater than 50% with androgen annihilation, as shown in the waterfall plot and table below.
In an exploratory analysis of biomarkers of response, tumors classified as luminal by the PAM50 signature score, or those having high AR activity expression signatures trended towards improved rPFS and overall survival with androgen annihilation.

With regards to safety, the profile of androgen annihilation was as would be expected from the cumulative effects of both drugs, with no new safety signals. Based on FACT-P score, QoL was similar between each group.

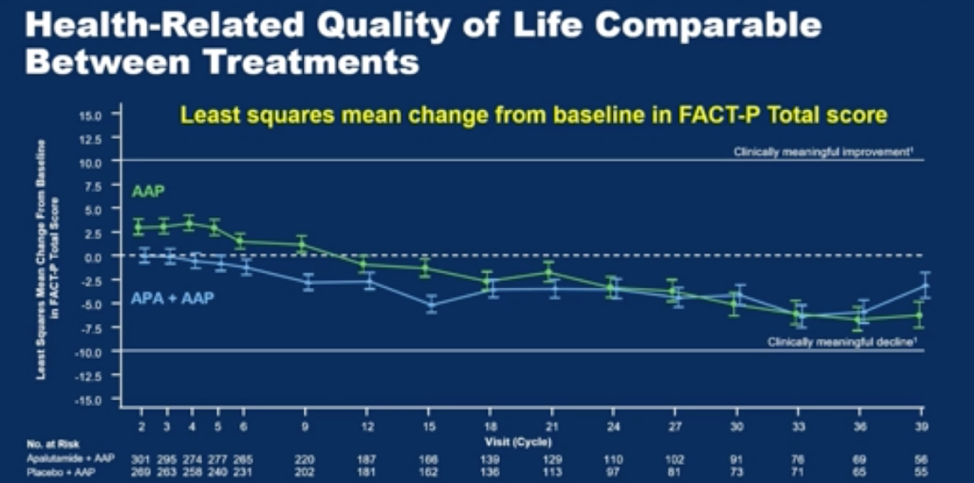
Dr. Rathkopf concluded that the ACIS trial met its primary outcome of showing an rPFS benefit for abiraterone plus apalutamide as first line therapy in mCRPC, but the secondary endpoint of overall survival and others were similar between the treatment arms. While more treatment related adverse events were noted in the combination arm, the overall QoL as measured by FACT-P was similar between arms.
Presented by: Dana Rathkopf, MD, Medical Oncologist, Memorial Sloan-Kettering Cancer Center,
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 ASCO Genitourinary Cancers Symposium (ASCO GU), February 11th to 13th, 2021


