(UroToday.com) In this presentation, Dr. de Bono summarized results of the IPATential150 trial, previously presented at ESMO 2020, which demonstrated an rPFS benefit for the combination of the AKT-inhibitor ipatasertib with abiraterone in patients with metastatic castration-resistant prostate cancer (mCRPC). This benefit was seen in the intention to treat population as well as when patients were stratified by PTEN expression status as defined by immunohistochemistry. PTEN loss was defined as more than 50% of viable malignant cells having no PTEN cytoplasmic staining using the SP218 PTEN immunohistochemistry antibody. The study design is summarized below.
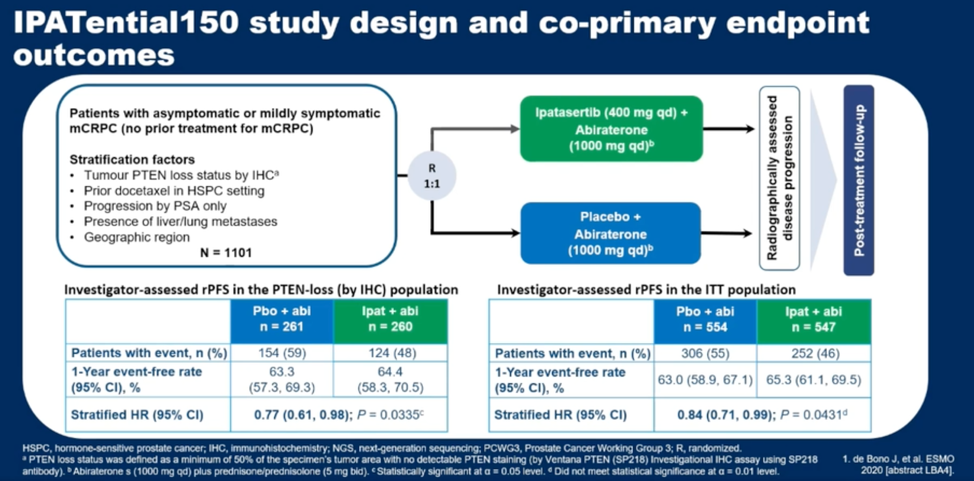
In this study, Dr. de Bono and colleagues evaluated several questions: (1) What is the impact of differential IHC PTEN loss status cutoffs on treatment efficacy, (2) What is the concordance between PTEN assessment by immunohistochemistry and next generation sequencing (NGS), (3) How does PTEN loss by next generation sequencing relate to outcomes, and (4) How do other genomic alterations in the PI3K/AKT pathway associate with treatment outcomes with ipatasertib.
When stratifying patients by varying cutoffs to define PTEN loss by IHC, all thresholds of some PTEN expression loss defined patients that tended to benefit from ipatasertib combination therapy. The trend improved at PTEN loss thresholds of greater than 50%, as illustrated below. 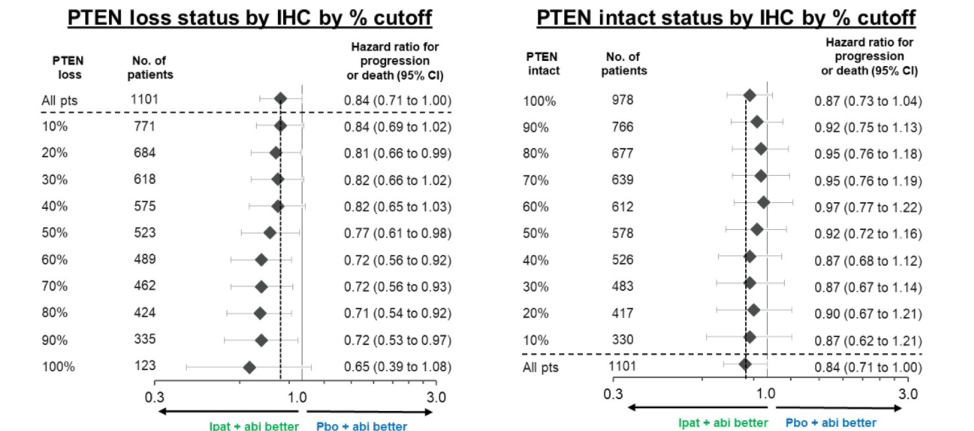
To answer the remaining questions, Dr. de Bono first illustrated the NGS analysis flowchart used by the investigators, reproduced below. Out of 1101 patient samples, 67% were evaluable by NGS, and 70% of these were able to be evaluated for PTEN status. 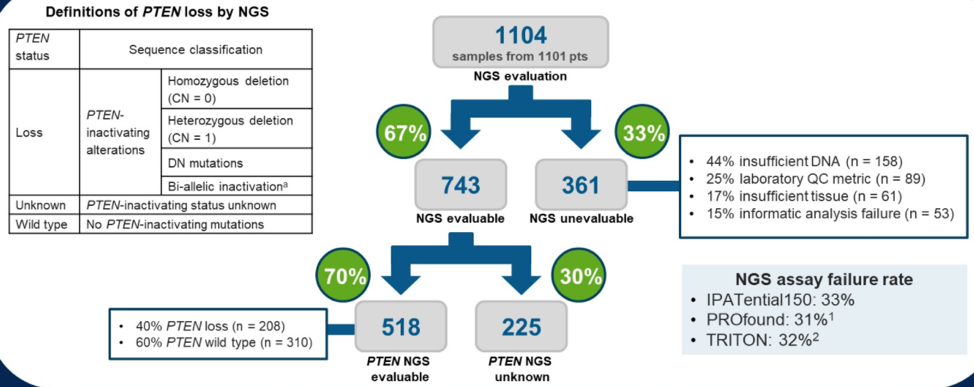
The overall agreement between PTEN status by IHC and NGS was 85.5%. Of the 208 samples with PTEN loss by NGS, 91.3% also showed PTEN loss by IHC. Among the 247 samples with PTEN loss by IHC, 190 had PTEN loss by NGS. Importantly, stratifying patients by PTEN loss as assessed by NGS confirmed the rPFS benefit for combination ipatasertib with abiraterone (HR 0.65, 95% CI 0.45-0.95). Using NGS to expand the biomarker selection to other alterations in PI3K/AKT/PTEN signaling also showed rPFS improvement with combination ipatasertib and abiraterone. 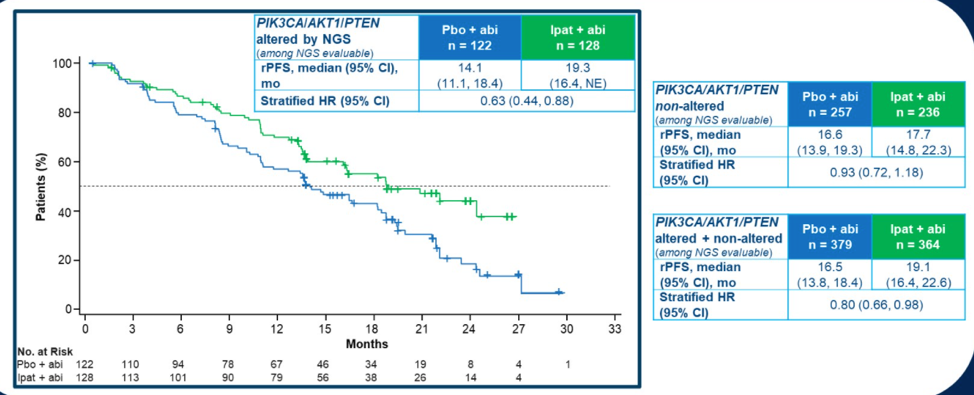
In summary, Dr. de Bono concluded that PTEN assessment by IHC and NGS had good concordance and that some patients with PTEN loss have other PI3K and AKT gene alterations. He stated that his results support the use of ipatasertib with abiraterone as a treatment option for first-line therapy in mCRPC patients with PI3K/AKT/PTEN pathway alterations.
Presented by: Johann de Bono, MB ChB FRCP MSc Ph.D. FMedSci. Professor Johann de Bono is Regius Professor of Cancer Research and a Professor in Experimental Cancer Medicine at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust.
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021
Related Content:
ESMO Virtual Congress 2020: IPATential150: Phase III Study of Ipatasertib plus Abiraterone vs Placebo plus Abiraterone in Metastatic Castration-Resistant Prostate Cancer
IPATential150: Phase III Study of Ipatasertib plus Abiraterone vs Placebo plus Abiraterone in Metastatic Castration-Resistant Prostate Cancer - Cora Sternberg


