In prostate cancer, drugs targeting the activated PI3K signaling pathway have shown limited activity in advanced disease. This is due to a combination of dose-limiting toxicities, functional redundancies, complex compensatory mechanisms, and challenges with appropriate biomarker selection. Importantly, the androgen receptor and PI3K signaling pathways cross-regulate each other. This has been shown in multiple publications and is illustrated in the schema below. This cross-regulation suggests an opportunity for dual inhibition of AR and PI3K signaling.
Two pan-AKT inhibitors (ipatasertib and capivasertib) that target a critical junction of PI3K signaling have been studied in advanced prostate cancer with promising results. The combination of ipatasertib with abiraterone was shown to improve rPFS in patients with PTEN loss by immunohistochemistry. The combination of capivasertib with docetaxel chemotherapy in the ProCAID trial, however, did not improve progression-free survival regardless of PTEN loss status. Interestingly, an overall survival advantage was seen with capivasertib, though the reasons for this are unclear, and this finding requires prospective validation. These phase 2 results led to the design of the phase 3 IPATential150 combination trial of ipatasertib and abiraterone in mCRPC, with the schema shown below.
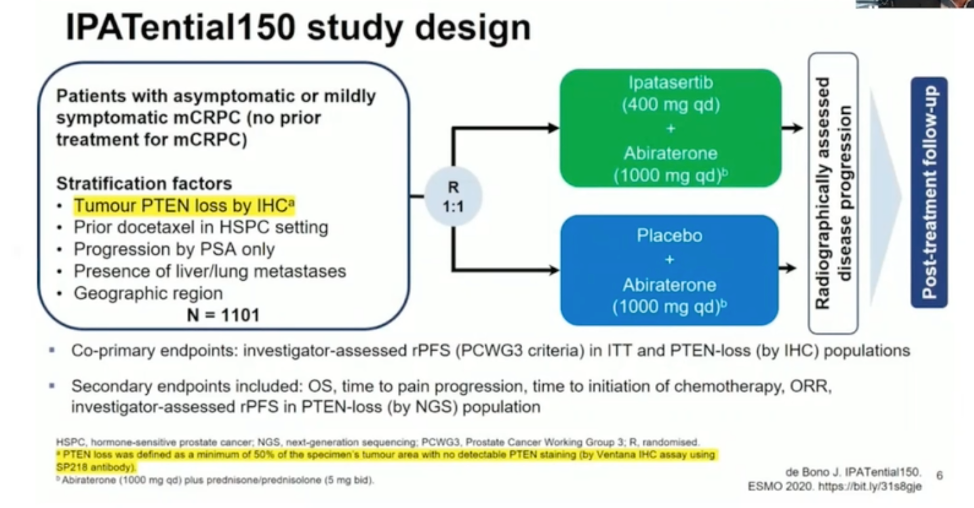
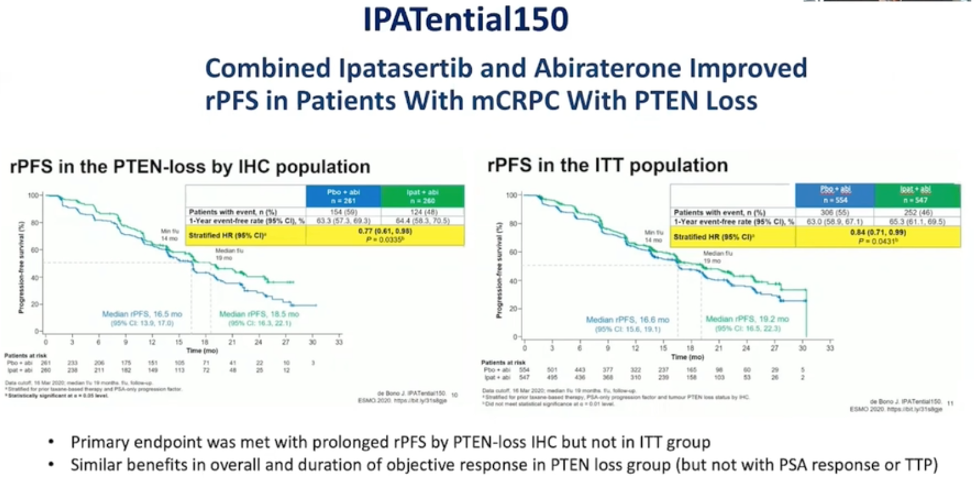
As shown below and presented at ESMO 2020, the combination improved rPFS to the specified threshold in the PTEN-loss mCRPC patient population. Overall survival data are not yet mature. Combination therapy was associated with more treatment-related adverse events such as rash, diarrhea, and hyperglycemia.
The question of the best assay to define PI3K activation remains and is discussed further in Abstract 126. Both IHC and next-generation sequencing have limitations. Immunohistochemistry is cheap, feasible, specific, is useful in cases of homozygous loss, and can capture non-genomic causes of loss of PTEN expression. However, IHC misses other important PI3K pathway alterations, can be subject to arbitrary technical issues and cutoffs, and cannot detect loss of function alterations where protein expression remains intact. Next-generation sequencing (NGS) can detect other key genomic alterations and better delineate the genomic context of the tumor (ie co-deletion of MYC or TP53) but is limited in that many tumors fail nucleic acid extraction and sequencing. Circulating tumor DNA may also be a feasible way to select patients with PI3K signaling activation.
Many caveats exist regarding the combination of PI3K and androgen receptor co-targeting. While mCRPC often harbors amplification of the AR locus, is this combination likely to be effective in samples with lower levels of AR alterations? When might ipatasertib monotherapy be useful? Some preclinical data suggests that ipatasertib has significant monotherapy activity in the context of AKT1 activating mutations.
Dr. Gleave concluded his talk, summarizing that PI3K pathway activation via multiple mechanisms is common in prostate cancer. Randomized trials of AKT inhibitors with abiraterone have shown some clinical benefit, but also significant toxicity. Ongoing work is required to determine optimal criteria for selecting patients that will benefit the most from dual AR and PI3K pathway inhibitions. Examples of ongoing trials include the CCTG Umbrella Trial (NCT03385655), the RE-AKT phase 2 trial of enzalutamide with or without capivasertib in mCRPC (NCT02525068), and the CAPItello-281 trial of abiraterone with or without capivasertib in mHSPC (NCT04493853).
Presented by: Martin Gleave, MD, FRCSC, FACS, University of British Columbia
Written by: Alok Tewari, MD, Ph.D., Medical Oncologist at the Dana-Farber Cancer Institute, during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2020


