
To this end, in a plenary abstract presentation in The Clinical Conundrums of Genomically Driven Prostate Cancer session at the 2021 ASCO GU Cancers Symposium, Dr. Rana McKay and colleagues present work examining the combination of cediranib, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, and olaparib.
Cediranib is an oral ATP-competitive tyrosine kinase inhibitor of VEGF receptor 1-3. Anti-angiogenic agents such as cediranib can induce a hypoxic tumor environment which results in downregulation of homologous recombination genes. Thus, there is both a rationale, as well as preclinical data, to support the combination of cediranib and olaparib.

The authors recruited men with mCRPC who had received at least one prior line of systemic therapy in this disease state. Eligible men were randomized in a 1:1 fashion to olaparib 200 mg by mouth twice daily with cediranib 30 mg by mouth daily or monotherapy olaparib (300 mg by mouth twice daily). Included patients underwent baseline biopsy of a metastatic site for histologic confirmation with next-generation sequence of HRR genes using the BROCA-HR assay. Notably, patients were not required to have HRR deficiency however, patients were defined as having HRR deficiency if they had bi-allelic loss in HR genes including BRCA1, BRCA2, ATM, and others. Patients were followed to assess the primary endpoint of radiographic progression-free survival (rPFS) and secondary endpoint of overall survival.

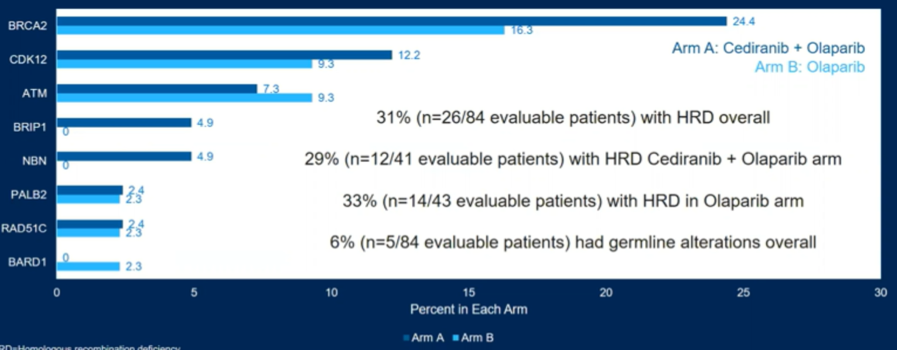
The authors included in their analysis 90 patients, with 84 being evaluable for HRR status of whom 26 patients (31%) had HRR deficient mCRPC.

The most common HR gene alterations included BRCA2 (n=17, 20%), CDK12 (n=9, 11%), and ATM (n=7, 8%).
Over a median follow-up of 10.98 months, assessing their primary outcome of rPFS in the overall cohort, the authors demonstrated a statistically significant improvement of 4.5 months (8.5 vs 4.0 months; hazard ratio 0.625, 95% CI 0.395-0.990) for patients receiving cediranib with olaparib as compared to olaparib alone. Notably, this effect was larger (though not statistically significant due to small numbers) among patients with HRR deficient disease.

Notably, in patients with HRR proficient disease, there was minimal difference in rPFS. Further, independent of randomized arm stratum, patients with HRR deficient disease had longer rPFS and overall survival compared to those with HRR proficient disease, though neither comparison was statistically significant (rPFS: 8.8 versus 4.3 months, p=0.14; OS: 18.6 vs. 12.3 months, p=0.24).
The authors further explored rPFS by individual gene mutations. While overall numbers were low limiting definitive conclusions, the combination approach provided benefit in many of these patient groups.

Further analysis of secondary endpoints demonstrated non-significantly longer median overall survival in the olaparib arm (17 months) compared to the combination arm (12 months; p=0.4). Notably, 13 of 45 patients in olaparib alone crossed over at the time of radiographic progression. PSA responses and objective response rates were higher in the combination arm. However, the combination approach was associated with increased toxicity, including higher rates of dose reductions and discontinuation due to toxicity.
The authors conclude that the combination of receiving cediranib with olaparib, as compared to olaparib alone, confers a benefit in rPFS for patients with mCRPC, with the effect driven by patients with HRR deficient tumors.
Presented by: Rana R. McKay, MD, Medical Oncologist, Assistant Professor of Medicine, UC San Diego Health
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter during the 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium (#GU21), February 11th-February 13th, 2021


