(UroToday.com) Use of adjuvant therapy intrinsically will result in a subset of patients receiving unnecessary treatment, making the appropriate selection of patients for adjuvant treatment critical. CheckMate 274, which represented a step forward for adjuvant treatment in high-risk muscle-invasive bladder cancer (MIBC), demonstrated improved disease-free survival with the use of adjuvant nivolumab. The authors of this study sought to evaluate the appropriateness of inclusion criteria in CheckMate 274 by retrospectively evaluating the outcomes for patients who met and did not meet eligibility criteria for nivolumab within the trial.
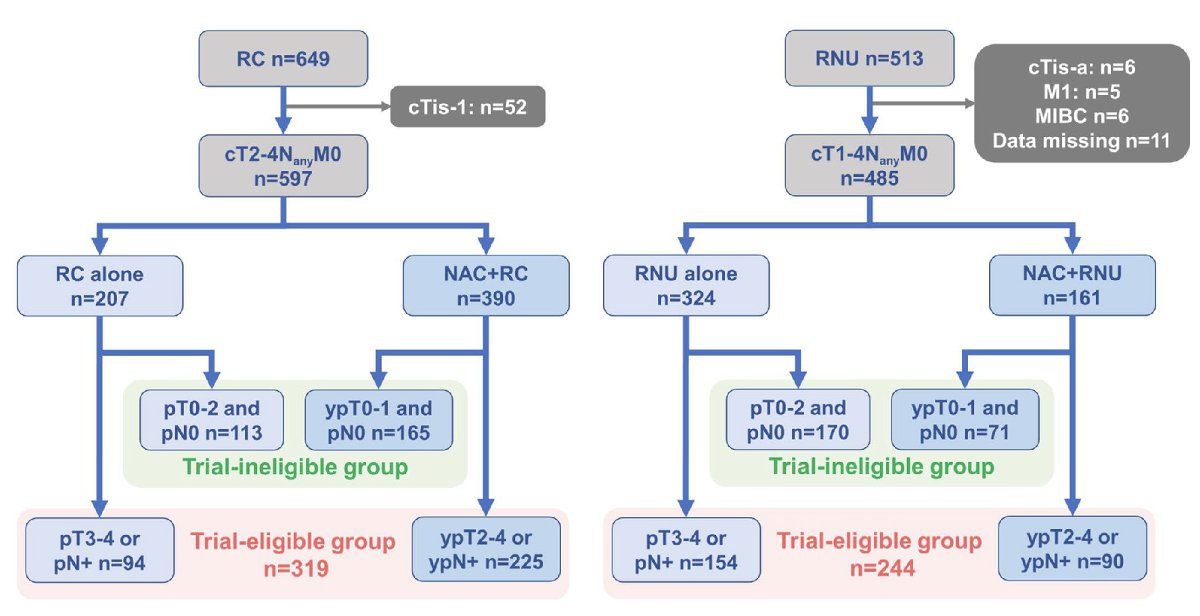
1082 patients with either localized and locally advanced MIBC and upper tract urothelial carcinoma (UTUC) were evaluated following cystectomy (n=597) or nephroureterectomy (n=485), respectively, performed between January 2000 and April 2021. The median age was 69 and 72 years for each disease, respectively. As guided by the CheckMate 274 criteria (postoperative pT3-4/ypT2-4 or pN+), the authors patients into those patients that met those criteria and those who did not. The primary outcome was the effect of meeting trial eligibility criteria on disease-free survival (DFS) and overall survival (OS). Secondary outcomes included the changes to prognostic and risk model development when lymphovascular invasion (LVI) status was added to trial criteria.
The authors report that 52% of patients met the trial inclusion criteria and these patients had worse DFS and OS in both tumor types, as shown.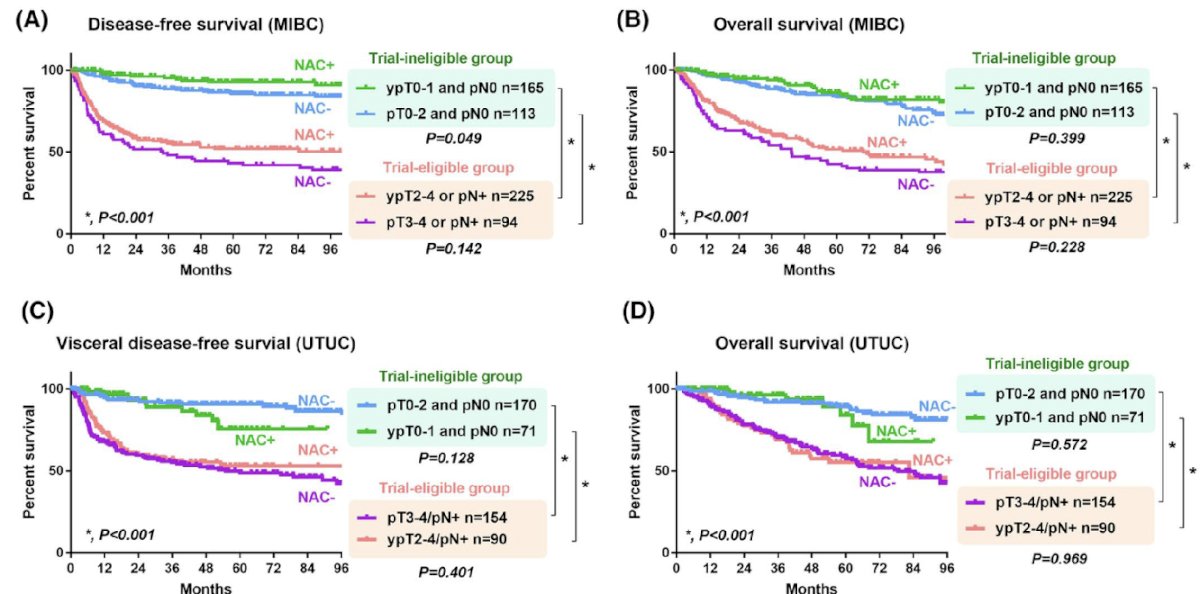
The addition of LVI presence to trial criteria was further associated with a poorer prognosis. Those patients with LVI+ or pN+ among those with pT3-4 or ypT2-4 were found to be at the very highest risk. The authors' data appear to support the CheckMate 274 established criteria for patient selection for adjuvant nivolumab, although additional sensitivity or specificity could be explored. The addition of LVI, based on these data, may provide additional confidence to a treating medical oncologist when opting to offer nivolumab in such a patient in the neoadjuvant setting.
Presented By: Shingo Hatakeyama, MD, Hirosaki University, Hirosaki, Japan
Written By: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2022 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 17 – Sat, Feb 19, 2022.
References:


