(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma session featuring work from Dr. Tanya Jindal and colleagues presenting work assessing biomarkers predictive of response to enfortumab vedotin treatment in advanced urothelial cancer. Enfortumab vedotin is an antibody-drug conjugate that recently received full FDA approval for treatment-refractory advanced urothelial cancer. Molecular biomarkers and characteristics of patients most likely to respond to enfortumab vedotin therapy have not been well defined.
This study retrospectively identified all advanced urothelial cancer patients treated with enfortumab vedotin at the University of California San Francisco. Clinicopathologic, treatment, and response data were abstracted from patient charts. Patients were considered responders to enfortumab vedotin if they had a complete response on initial scans after 2-3 months of treatment, or were treated with enfortumab vedotin for ≥ 6 months. Responders and non-responders were compared in terms of their molecular and clinical characteristics using Chi-squared test. Most common somatic alterations present in ≥10 patients (TERTp, TP53, CDKN2A, CDKN2B) were also used to divide patients with available next-generation sequencing (NGS) results into groups with and without these alterations. Log rank test was used to determine differences in overall survival (OS) and progression free survival (PFS) among these groups.
Between January 2020 and August 2021, a total of 32 patients received enfortumab vedotin and 28 had NGS data available with either FoundationOne (14 patients), UCSF500 (13 patients) or Strata (1). The median age was 69.5 years, 24 (75%) were male, 22 (69%) Caucasian, 22 (69%) had pure urothelial histology, and 22 (69%) primary tumor location in the bladder. At the start of enfortumab vedotin, 24 (75%) had visceral metastases, 8 (25%) had liver metastases, and 13 (41%) had bone metastases. Median follow-up from enfortumab vedotin start was 12.5 months (range 0.5-36), 20 (63%) patients received enfortumab vedotin monotherapy, and 12 (37%) received enfortumab vedotin as part of a combination regimen. A summary of the baseline characteristics is as follows:
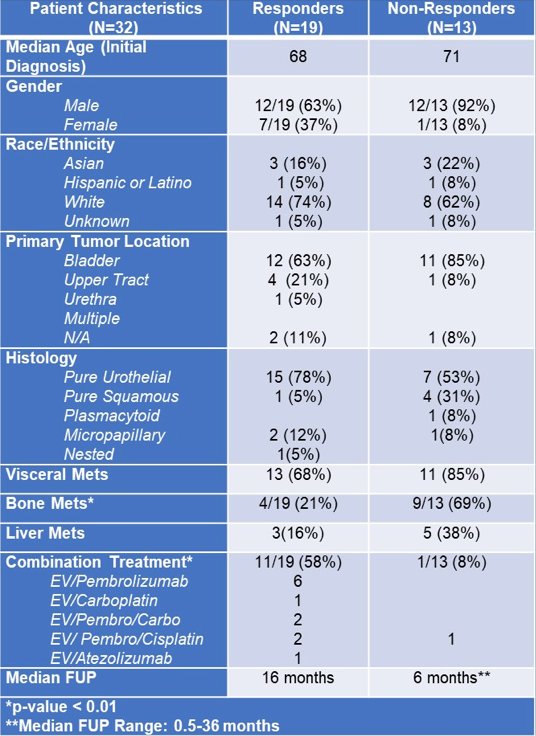
Non-responders were more likely to have bone metastases (69% vs 21%, p<0.01), but were otherwise similar in baseline clinical characteristics to responders. TP53 alterations were enriched in responders relative to non-responders, whereas non-responders had more CDKN2B alterations:

Similar findings were seen in the subset of patients treated with enfortumab vedotin monotherapy. Patients with TP53 alterations had longer OS (not reached vs 17.0 months, p=0.06) and PFS (not reached vs 6.6 months, p=0.04) relative to wild-type patients:

Shorter PFS was seen in patients with CDKN2A (4.4 months vs not reached, p=0.05) and CDKN2B (4.3 months vs not reached, p=0.02) alterations, but no differences in OS were observed.
Dr. Jindal concluded her presentation of biomarkers predictive of response to enfortumab vedotin for the treatment of advanced urothelial carcinoma with the following take-home messages:
- The presence of TP53 and absence of CDKN2A and CDKN2B alterations were associated with favorable responses and improved clinical outcomes with enfortumab vedotin, suggesting they may be biomarkers of response to enfortumab vedotin
- A plausible mechanism explaining these findings is that CDKN2A alterations may be mutually exclusive with TP53 alterations and also with Nectin-4 amplification, which is necessary for enfortumab vedotin mechanism of action
- These preliminary findings are hypothesis generation and should be validated in larger cohorts, but can potentially inform clinical decision making for patients with advanced urothelial carcinoma being considered for treatment with enfortumab vedotin
Presented By: Tanya Jindal, University of California San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Co-Authors: Li Zhang, Jonathan Chou, David Shui, Sima P. Porten, Anthony C. Wong, Emily Chan, Bradley A. Stohr, Ivan de Kouchkovsky, Hala Borno, Rohit Bose, Daniel H Kwon, Arpita Desai, Franklin W. Huang, Rahul Raj Aggarwal, Eric Jay Small, Lawrence Fong, Terence W. Friedlander, Vadim S Koshkin
Affiliations: University of California San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, San Francisco, CA, University of California San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, University of California San Francisco, San Francisco, CA, Department of Pathology, University of California, San Francisco, San Francisco, CA, University of California, San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, University of California, San Francisco, San Francisco, CA, UCSF, San Francisco, CA, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco, CA
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


