(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focused on urothelial carcinoma, in Poster Session B, Dr. Palak Kundu presented the safety profile of stereotactic body radiotherapy (SBRT) in combination with durvalumab (anti-PDL1) and tremelimumab (anti-CTLA4) for patients with cisplatin-ineligible, unresectable locally advanced or metastatic bladder cancer. Bladder preservation is increasingly recognized as an alternative to radical cystectomy for patients with localized muscle-invasive bladder cancer (MIBC), many of whom are not ideal candidates for either chemotherapy or radical cystectomy.
However, bladder preservation with maximal transurethral resection of bladder tumor (TURBT) and stereotactic body radiotherapy (SBRT) is a viable treatment option for such patients, offering durable local control while potentiating the effect of immunotherapy.
Thus, the authors accrued patients with locally advanced or metastatic urothelial carcinoma, who were unwilling or unable to undergo radical cystectomy and/or systemic chemotherapy (NCT03601455). Patients underwent maximal TURBT, followed by durvalumab 1500mg IV every 4 weeks and bladder SBRT 33Gy in 5 fractions between the first 2 cycles. After 6 patients treated in this manner did not experience a dose limiting toxicity, tremelimumab 75mg IV was added for cycles 1-2.
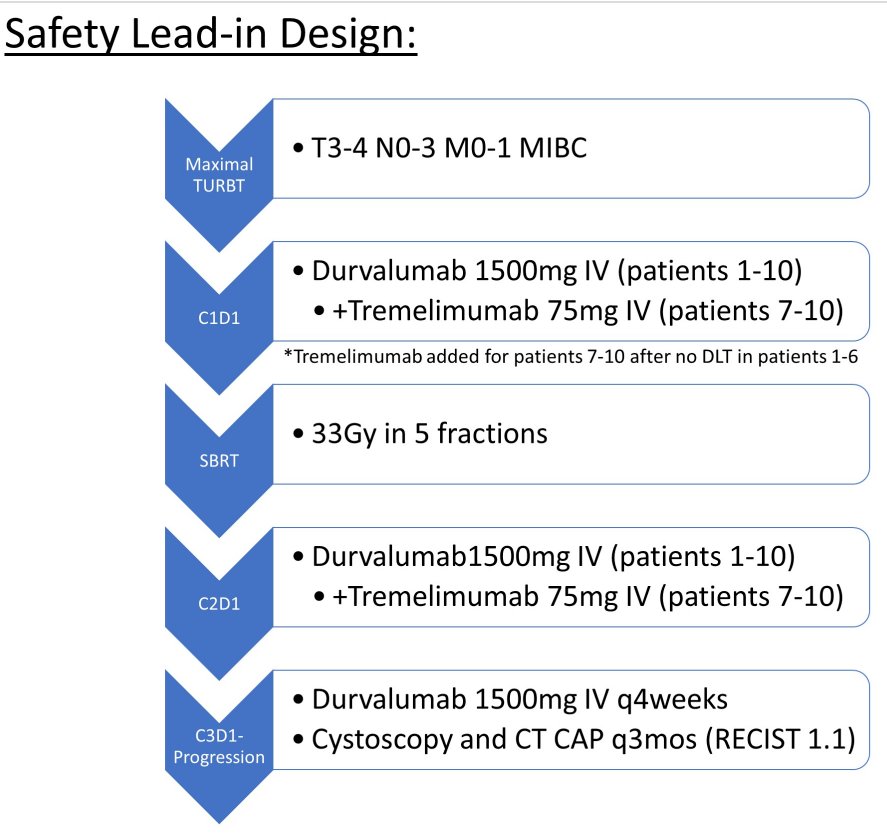
Patients were followed with CT of the Chest/Abdomen/Pelvis and cystoscopy every 3 months. Treatment-related adverse events (TRAEs) were assessed per CTCAE 5.0 and progression was assessed per RECIST 1.1.
Between January 2019 and May 2020, the authors identified, enrolled, and treatment 10 patients with TURBT followed by SBRT and durvalumab. Included patients ranged in age from 73 to 96years, 3 were Female, and 7 had ECOG performance status of 1. Five patients had localized disease and 5 had nodal or distant metastases. The median number of cycles of durvalumab was 12.5 (2-13), and 4 patients received 2 cycles of tremelimumab.
At a median follow-up of 16.8 months (range 6.4 to 30 months), there were 2 Grade 3+ immunotherapy-related TRAEs (1 Grade 3 lipase elevation which self-resolved, and 2 Grade 3 myositis events which were treated with steroids). The most common TRAEs of any grade included pancreatic enzyme elevation (3 patients), rash (3 patients), and urinary symptoms related to RT (3 patients). There were 3 non-treatment related deaths.
Disease control rate was 70% and local control was 90%.
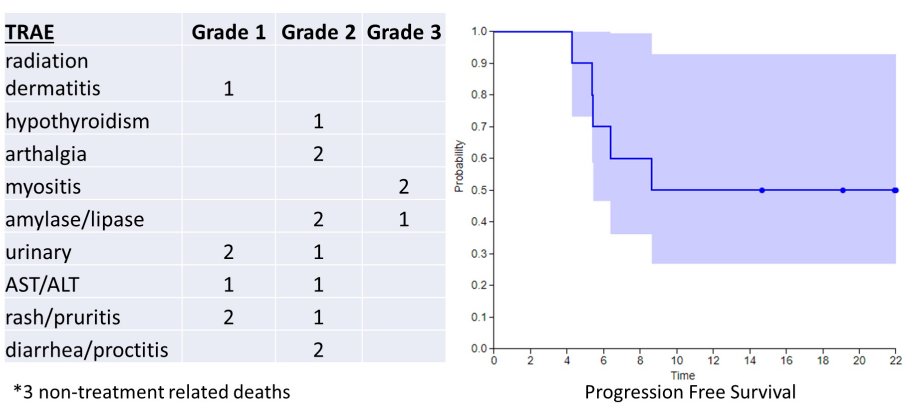
Thus, Dr. Kundu concluded that bladder SBRT with durvalumab and tremelimumab was well tolerated and demonstrated promising local control in the safety lead-in cohort among patients with MIBC who are unable or unwilling to undergo radical cystectomy or chemotherapy.
Presented by: Palak Kundu, MD, MBA, Department of Radiation Oncology, University of California, Los Angeles, Los Angeles, CA

