(UroToday.com) In an oral abstract presentation on the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focused on urothelial carcinoma, Dr. Rosenberg presented results of BAYOU, examining durvalumab and olaparib as first-line therapy in platinum-ineligible patients with unresectable stage IV urothelial carcinoma.
Patients with metastatic urothelial carcinoma have a poor prognosis, particularly among those who are not fit for platinum-based chemotherapy. In this setting, treatment options include atezolizumab and pembrolizumab, though there remains a significant unmet need. Aberrations in DNA damage repair (e.g., mutations in homologous recombination repair [HRR] genes) are common in patients with UC. These mutations render tumor cells sensitive to poly(ADP-ribose) polymerase (PARP) inhibition. Thus, the combination of HRR gene mutations (HRRm) and PARP inhibition may enhance the antitumor response of immune checkpoint inhibitors.
BAYOU is a randomized phase II study to evaluate durvalumab (an anti–PD-L1 agent) in combination with olaparib (a PARP inhibitor) or placebo as a first-line treatment for platinum-ineligible pts with unresectable, stage IV UC (NCT03459846).
The authors enrolled patients aged 18 years and older with an ECOG performance status 0-2 and histologically or cytologically confirmed transitional cell carcinoma who had not received prior systemic therapy for unresectable, stage IV disease. Patients were randomized in a 1:1 fashion to receive durvalumab (1500 mg IV q4w) plus olaparib (orally at 300 mg BID) or durvaluamb (1500 mg IV q4w) plus olaparib-matching placebo. Randomization was stratified according to centrally-determined HRR status (mutant vs wild-type) and Bajorin risk index (a composite of visceral metastases and ECOG PS [0, 1 vs 2]).

The primary endpoint was progression-free survival (PFS) by RECIST v1.1 (investigator assessed) in the intention-to-treat (ITT) population with additional secondary endpoints including overall survival (OS) in the ITT population and PFS in the subset of patients with HRRm.
Among 154 patients who were enrolled, 78 were randomized to receive durvaluamb and olaparib while 76 were randomized to durvaluamb and placebo. Approximately two-thirds of the study population had visceral metastasis. Notably, 22% of patients in the durvaluamb and olaparib group and 18% of those in the durvaluamb and placebo group had HRR mutations. Tumor samples were assessed for loss of function alterations in one of fifteen pre-specified HRR genes using the validated FMI FoundationOne assay.

As of a data cut-off of October 15, 2020, median PFS did not significantly differ between those patients treated with durvaluamb and olaparib (median 4.2 months) and those treated with durvaluamb and placebo (median 3.5 months) in the ITT population (hazard ratio 0.94, 95% CI 0.64 to 1.39).

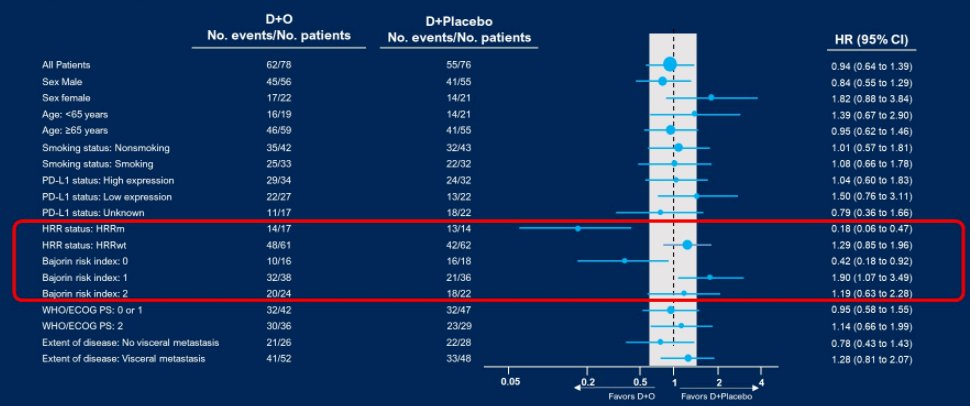
Among the subset of patients with HRR mutations, the median PFS was 5.6 months in the durvaluamb and olaparib group and 1.8 months in the durvaluamb and placebo group.

Further subgroup analyses demonstrated effect modification by both HRR mutation status and Bajorin risk score.
In the ITT population, median OS (95% CI) was 10.2 months (7.0–13.9) in the durvaluamb and olaparib group and 10.7 months (7.2–17.3) in the durvaluamb and placebo group (HR 1.07, 95% CI 0.72–1.61). In the HRR wild-type subset, patients in the durvaluamb and placebo group had longer median overall survival (13.7 months) than those in the durvaluamb and olaparib group (10.9 months, hazard ratio 1.35, 95% CI 0.85 to 2.16). In contrast, among the MRR mutant subset, median overall survival was longer among those receiving durvaluamb and olaparib (8.6 vs 5.8 months) though this was not significantly different (hazard ratio 0.56, 95% CI 0.25 to 1.23).

In terms of anti-tumor activity, objective response rates were higher among patients treated with durvaluamb and olaparib in both the ITT population (28% vs 18%) and in the HRR mutant subset (35% vs 0%).
Among all treated patients, grade 3 or 4 treatment-related adverse events occurred in 18% and 9% in the durvaluamb and olaparib and durvaluamb and placebo groups, respectively, with one death due to anemia in the durvaluamb and placebo group.

The authors therefore concluded that, based on the primary endpoint, durvaluamb and olaparib did not significantly prolong PFS, compared to durvaluamb and placebo in patients with previously untreated, platinum-ineligible mUC. However, pre-planned secondary, subset analyses suggested a particular benefit to this treatment regime in patients with HRR mutations.
Presented by: Jonathan E. Rosenberg, MD, Memorial Sloan Kettering Cancer Center


