(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma trials in progress session featuring SGNTUC-019, a phase 2 basket study of tucatinib and trastuzumab in previously treated solid tumors with HER2 alterations, presented by Dr. Matt Galsky. Tucatinib, a highly selective HER2-directed tyrosine kinase inhibitor approved in multiple regions for HER2+ metastatic breast cancer, is being investigated as a novel therapy for metastatic colorectal cancer, gastric cancer, and other GI tumors. The tucatinib proposed mechanism of action is as follows:
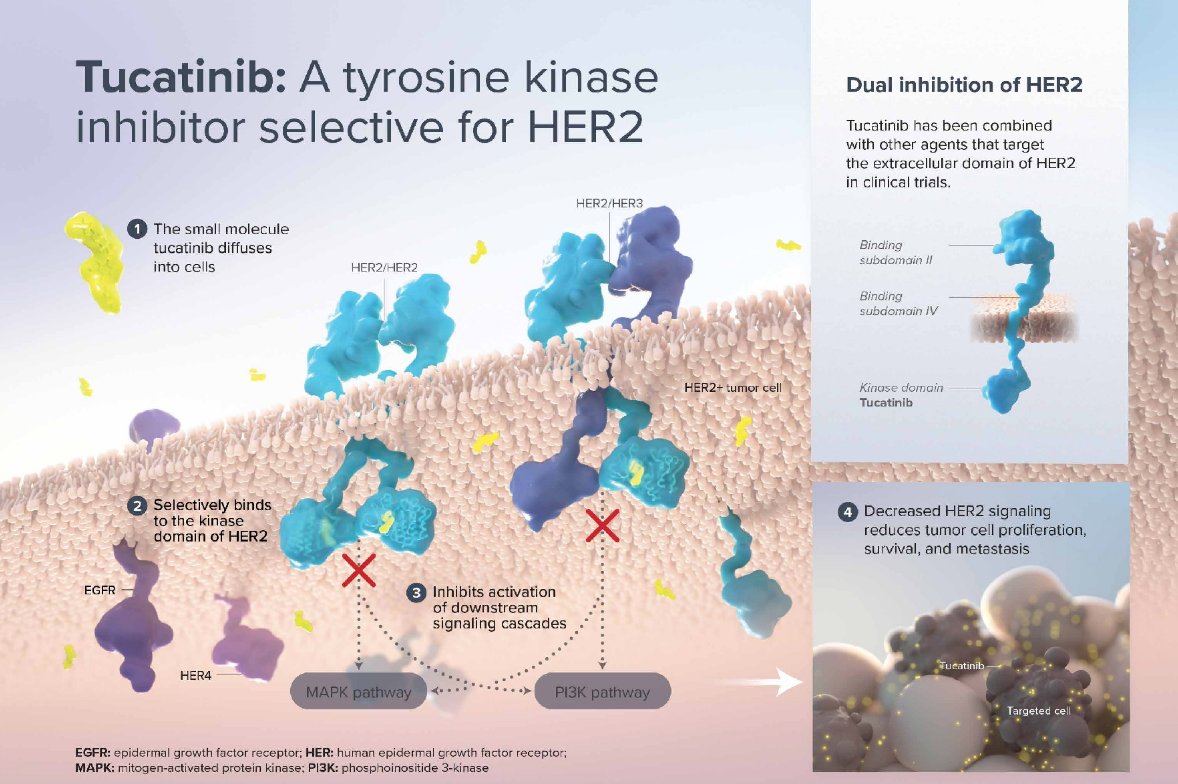
In xenograft models of HER2+ and HER2-mutated tumors, dual targeting of HER2 with tucatinib and trastuzumab showed superior activity to either alone. Despite development of several new therapies for metastatic urothelial cancer, most patients do not respond to subsequent therapies, and majority of patients succumb to the disease, highlighting the need for additional therapeutic approaches. Given 20%-30% of metastatic urothelial cancers have molecular alterations of the ErbB family, tucatinib + trastuzumab warrants further evaluation.
SGNTUC-019 (NCT04579380) is a multi-cohort, open-label, international Phase 2 study evaluating tucatinib + trastuzumab in patients with previously treated solid tumors displaying HER2+ or HER2-mutated solid tumors, including a cohort with locally advanced or metastatic urothelial cancer. Eligible patients must have HER2+ or HER2-mutated locally advanced or metastatic solid tumors, with progression on or after the last systemic therapy for advanced disease. Additionally, patients must have an ECOG PS ≤1, adequate hepatic, hematologic, renal, and cardiac function, and no prior exposure to HER2-directed therapy in the urothelial cancer cohort. For eligibility, HER2 alterations can be demonstrated by HER2 overexpression/amplification in tumor tissue by prior IHC/ISH, or by HER2 amplification/mutation in prior or prescreening NGS assay of ctDNA or prior tissue NGS assay.
The HER2 overexpression/amplification urothelial cancer cohort will enroll 12 response-evaluable patients per RECIST 1.1. If ≥2 responses are observed, the cohort will be expanded to 30. Patients with HER2-mutated urothelial cancer will be enrolled into a cohort of 30 patients with HER2mutated solid tumors (except breast and non-squamous nonsmall cell lung cancers). The study schema for SGNTUC-019 is as follows:
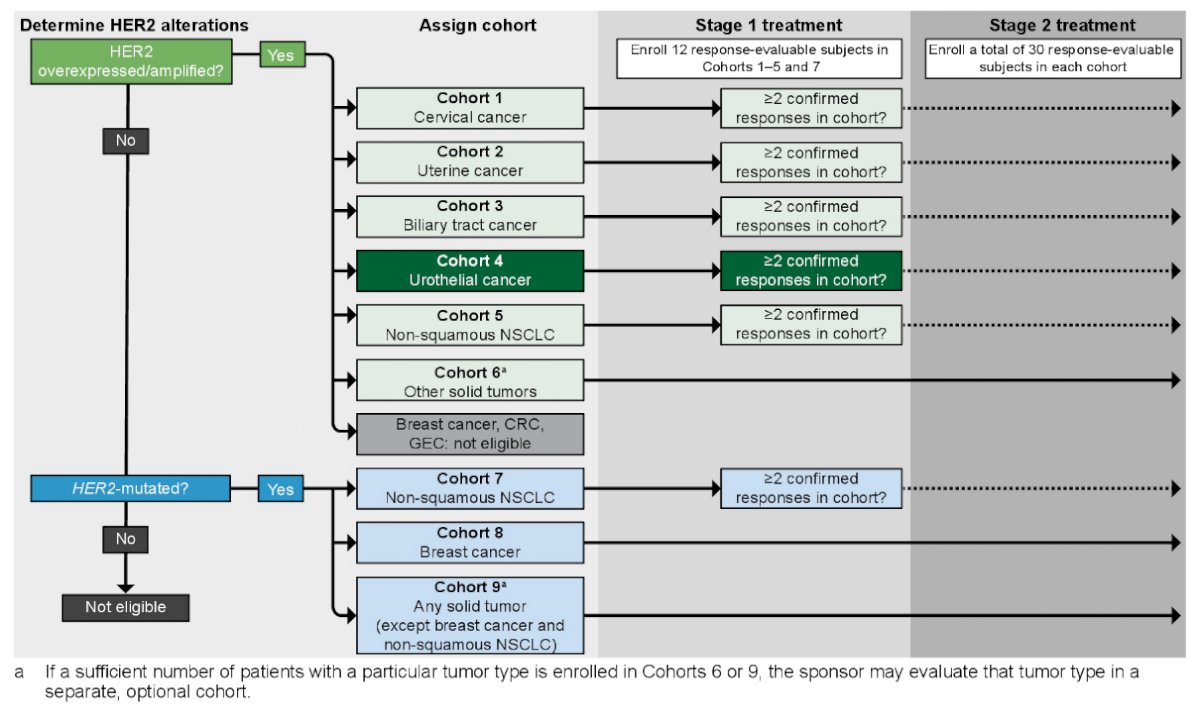
The primary objective is antitumor activity, with confirmed ORR as the primary endpoint, and disease control rate, duration of response, PFS, and OS as secondary efficacy endpoints. Patients will receive tucatinib 300 mg orally twice daily and trastuzumab 8 mg/kg intravenous on Cycle 1 Day 1 and 6 mg/kg q21 days from Cycle 2 Day 1. Disease assessments per RECIST 1.1 will occur q6 weeks for 24 weeks, then q12 weeks. Trough concentrations of tucatinib will be evaluated in Cycles 2-6, with a peak concentration sampled in Cycle 3:
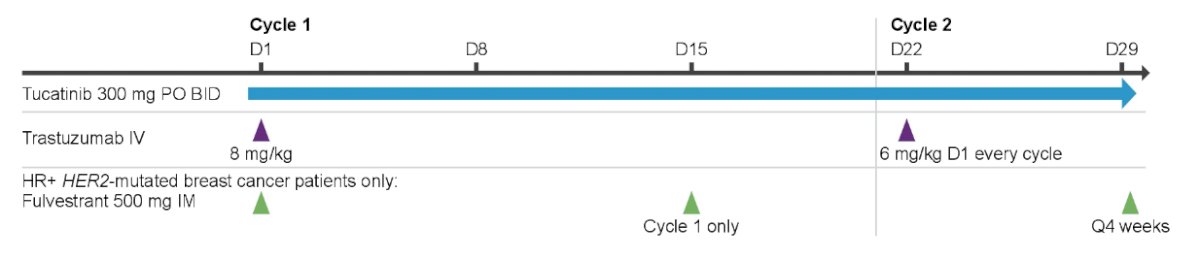
Quality of life will be evaluated q2 cycle using EQ-5D-5L. Sites are currently enrolling within the US, European Union, and the Asia Pacific.
Presented By: Matt D. Galsky, MD, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY
Co-Authors: Jorge Ramos, Qianwen Tan, Evan Y. Yu, Affiliations: Seagen Inc., Bothell, WA, University of Washington, Seattle, WA
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


