(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 Dr. Vulsteke presented the rationale and design of the TROPiCS-04 study of third-line sacituzumab govitecan in patients with locally advanced or metastatic urothelial carcinoma (mUC) during the Trials in Progress Poster Session B.
For eligible patients with locally advanced unresectable or mUC, first-line therapy is platinum-based chemotherapy and second-line therapy is immune checkpoint inhibitor therapy. While these agents have proven survival benefits, many patients will recur or progress and treatment options are limited following these two lines of therapy. Sacituzumab govitecan antibody-drug conjugate (ADC) composed of an anti-trophoblast cell surface antigen 2 (Trop-2) antibody coupled to SN-38 (a topoisomerase-I inhibitor) via a proprietary hydrolyzable linker. In the phase 2 registrational study TROPHY-U-01 (NCT03547973), sacituzumab govitecan demonstrated an objective response rate (ORR) of 27% and median overall survival (OS) of 10.9 months in patients with mUC who progressed after prior platinum-based chemotherapy and immune checkpoint inhibitors therapies. These data compare favorably to historical controls and formed the basis of accelerated approval by the US Food and Drug Administration. However, the phase 3 TROPiCS-04 trial (NCT04527991), a global, multicenter, open-label, randomized, controlled trial, was designed to confirm these findings.
TROPiCS-04 is enrolling patients with locally advanced unresectable or mUC who progressed after prior platinum-based chemotherapy and immune checkpoint inhibitor. Further, patients must have Eastern Cooperative Oncology Group performance status 0-1, no prior immune checkpoint inhibitor or ADC therapy within 4 weeks of study drug initiation, no history of active interstitial lung disease or non-infectious pneumonitis; and adequate hematologic, hepatic, and renal function.

Patients will be randomly assigned 1:1 to receive sacituzumab govitecan 10 mg/kg intravenously (IV) on days 1 and 8 of 21-day cycles or single-agent treatment of physician’s choice chemotherapy (paclitaxel 175 mg/m2, docetaxel 75 mg/m2, or vinflunine 320 mg/m2 IV on day 1 of 21-day cycles) until progressive disease, unacceptable toxicity, or withdrawal of consent. Treatment beyond progressive disease may be permitted in patients deemed to be receiving clinical benefit per investigator assessment.
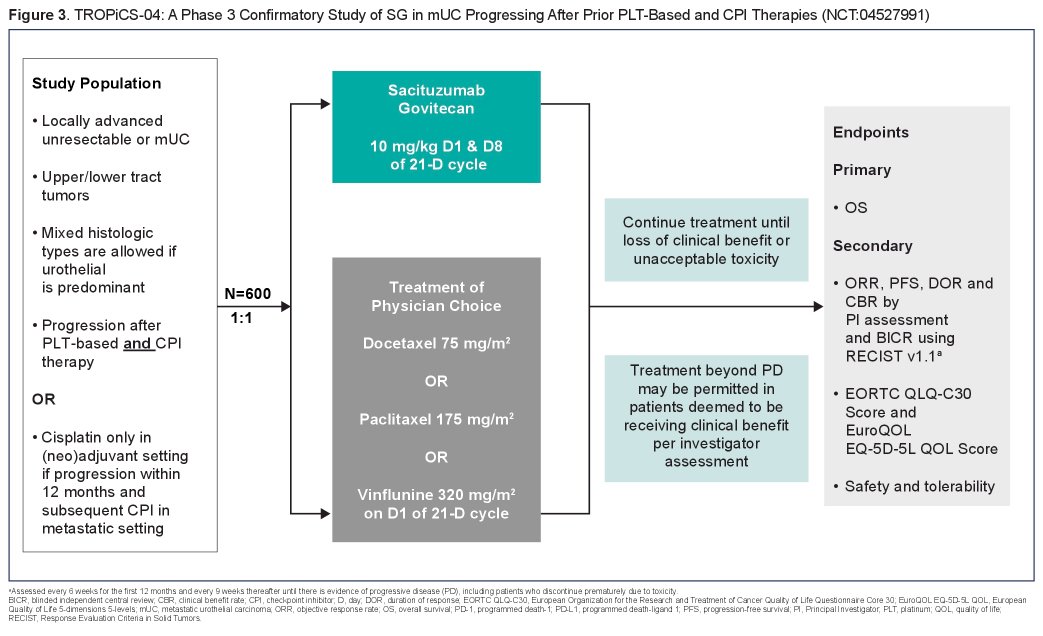
Beginning in January 2021, the authors plan to accrue approximately 600 patients across ~280 sites in North America, Europe, and Asia-Pacific. This sample size will provide 90% power on the primary endpoint of overall survival. In addition, secondary endpoints include progression-free survival, ORR, clinical benefit rate, duration of response (all per Response Evaluation Criteria in Solid Tumors version 1.1 by blinded independent central review and investigator assessment), safety, and quality of life.
Presented by: Christof Vulsteke MD, PhD, Integrated Cancer Center in Ghent, Maria Middelares, and Center for Oncological Research (CORE), University of Antwerp, Ghent, Belgium


