(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Dr. Tolcher presented in a session highlighting novel therapies in bladder cancer and their toxicities, beginning with the basic principles of tumor antigen targeting with antibody-drug conjugates. As the first presentation in this session, Dr. Tolcher began by highlighting the rationale for the development of antibody-drug conjugates, namely to optimize the specificity of cytotoxic drug to delivery both increasing the effectiveness of treatment and also avoiding normal tissue toxicity. He emphasized that, while effective, most systemic therapies have substantial off-target effects leading to significant toxicity.
The principle of antibody-drug conjugates was first conceived now nearly 30 years ago with a paper led by Dr. Trail published in Science which demonstrated the ability of BR96-doxorubicin immunoconjugates (comprising the chimeric monocloncal antibody BR96 linked to doxorubin) to cure approximately 70% of extensively metastatic xenografted human lung carcinomas in mice.
Dr. Tolcher then described the general principle of antibody-drug conjugates, emphasizing that these are complex molecular comprising an antibody which targets the cancer cell, a cytotoxic drug payload, and a link which joins the two.
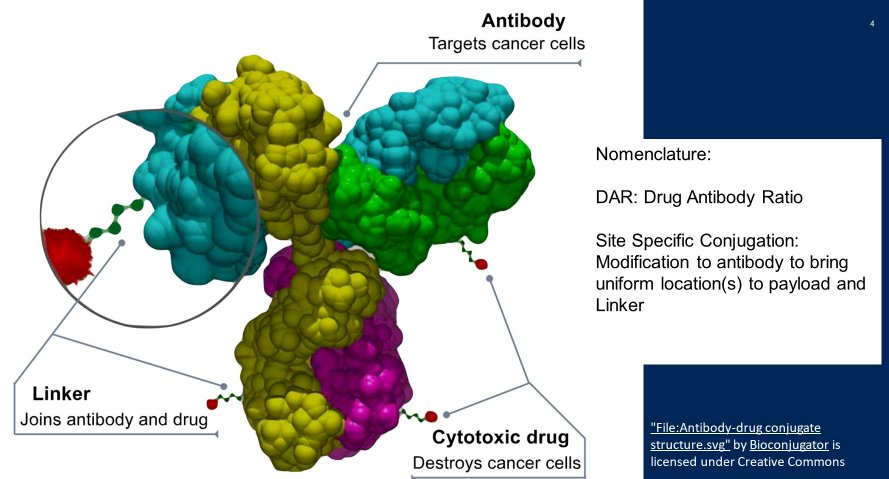
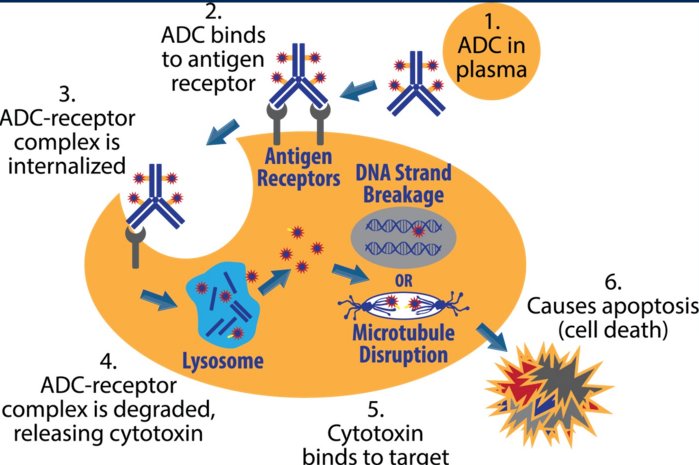
Mechanistically, antibody-drug conjugates (ADCs) for first infused and enter circulation. While circulating, on the basis of the antibody portion of the molecule, the ADC will then bind to an antigen receptor on the tumor cell of interest. This binding leads to internalization of the ADC and antigen receptor complex. Following internalization, lysosomal-mediated degradation of the ADC-receptor complex leads to release of the ADC’s cytotoxic payload. The cytotoxin then binds to its target. While mechanisms vary based on the specific payload used, either DNA strand breakage or microtubule disruption occurred leads to cellular apoptosis and death.
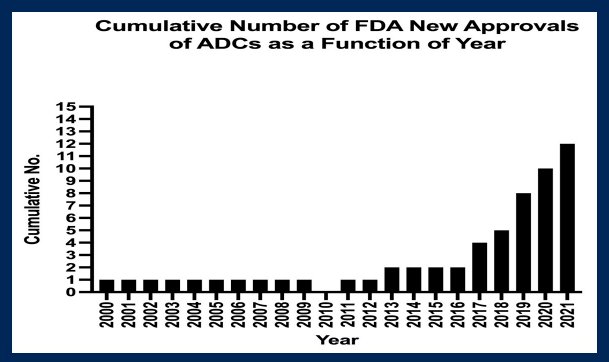
Building on the initial work from Trail and colleagues alluded to above, the first human trials of antibody-drug conjugates were performed using BR-96-doxorubicin. An initial phase I trial published in 2000 utilized this approach in patients with Lewis Y-expressing epithelial tumors. Subsequently, a randomized phase II study, comparing to doxorubicin was completed among women with metastatic breast cancer, led by Dr. Tolcher. Among 14 patients receiving the ADC BR96-doxorubicin, one partial response was noted though 1 complete response and 2 partial responses were seen in 9 patients receiving doxorubicin. While progress was subsequently slow, with a single FDA-approved ADC each year from 2000 to 2012 (with none in 2010), the cumulative number has begun to rise dramatically in the past few years and Dr. Tolcher highlighted that ADCs are now a major treatment platform in oncology.
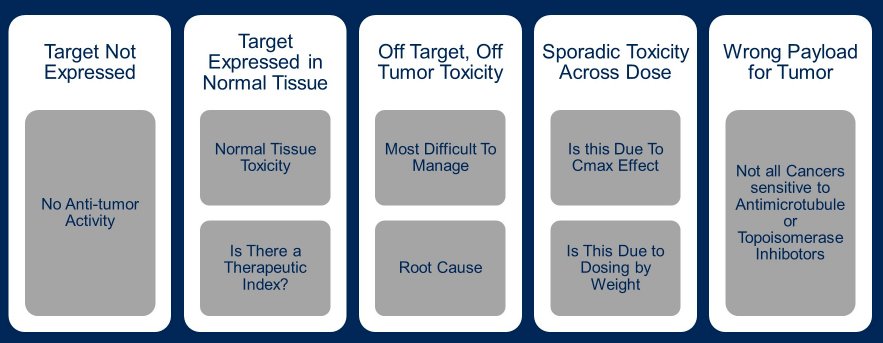
Dr. Tolcher then described the evolution of ADCs over time. Initially, there were significant effects of the targeting of the ADC to normal stomach tissue (when using BR96-doxorubicin). As a result, patients had significant nausea, vomiting, and hematemesis. Further, relatively weak linkers results in an early loss of the payload with resulting systemic toxicity. Additionally, the drug to antibody ratio was limited to 2 to 4. Moving forward from these early days, Dr. Tolcher highlighted the many things that can go wrong in the course of developing ADCs. First, the relevant target may be poorly expressed, in which minimal efficacy is observed. Alternatively, the target may be expressed in normal tissue resulting in toxicity. However, beyond this, there may be both off-tumor, off-target toxicity which can be difficult to manage and significant efforts are needed to understand the underlying causes of this phenomenon. Further considerations include a selection of the appropriate payload for a given tumor type as not all cancer are susceptible to antimicrotubule agents or topoisomerase inhibitors.
Working through these considerations, he emphasized the contemporary ADCs have better target selection, highlighting the example of 4-Nectin which is limited to urothelial cancer cells in the majority of patients with urothelial carcinoma, and is not found in normal issues. Further, there has been improvements in the chemistry underpinning the linkers, increasing their stability in plasma. There has further been evolution in site specific conjugation with a relatively narrow spectrum of drug to antigen ratio during the preparation process. Further, the drug to antigen ratio in the prepared product has increased to 8 or higher, increasing the potency.
Highlighting the examples of Sacituzumab Govitecan and DS106, he emphasized that not all ADCs will behave the same, even when they have the same target and similar payloads.

Moving forward, he suggested that ADCs will continue to evolve. Particularly, he emphasized that there can be a move from cytotoxics to targeted therapy as the payload. This is particularly useful as many targeted therapies, either alone or in combination, have significant toxicity when systemically administered. Further, immune stimulants are another potential payload. However, considering c’DOT technology, he raised the question of whether antibodies are even required.
In either case, he emphasized the ADCs have become a validated and accepted therapeutic platform, though they are not without meaningful toxicity.
Presented by: Anthony W. Tolcher, MD, FASCO, NEXT Oncology


