(UroToday.com) The 2022 GU ASCO Annual meeting included a session on the management of rare variants in genitourinary cancers and a presentation by Dr. Jonathan Rosenberg discussing the role of systemic therapy for the management of rare variant histology in urothelial cancer. Dr. Rosenberg notes that the optimal systemic therapy of variant histology in bladder cancer has not been defined. Mixed histology tumors are generally treated like conventional urothelial carcinoma (with some exceptions), with pure variants being rare entities. Trial accrual is difficult due to the rarity of these tumors, thus we must rely on data from case series, databases, expert opinion, and small single arm trials.
For small cell or high grade neuroendocrine carcinoma, early systemic therapy improves survival. Typical care includes chemoradiation with etoposide/platinum or neoadjuvant chemotherapy followed by radical cystectomy. There should also be considerations for neoadjuvant therapy among patients with focal small cell carcinoma in the setting of a mixed tumor and for T1 tumors in the localized setting.
Micropapillary urothelial carcinoma typically presents at higher stages at diagnosis, but stage for stage appears to have similar outcomes to urothelial carcinoma not otherwise specified. HER2 overexpression and amplification are enriched in micropapillary urothelial carcinoma, although overexpression and amplification are not tightly linked. Future HER2-directed trials should prospectively assess for presence of micropapillary disease.
Among patients with bladder adenocarcinoma, the genomic landscape suggests alterations in KRAS, PIK3CA, and TP53 (work submitted for publication):
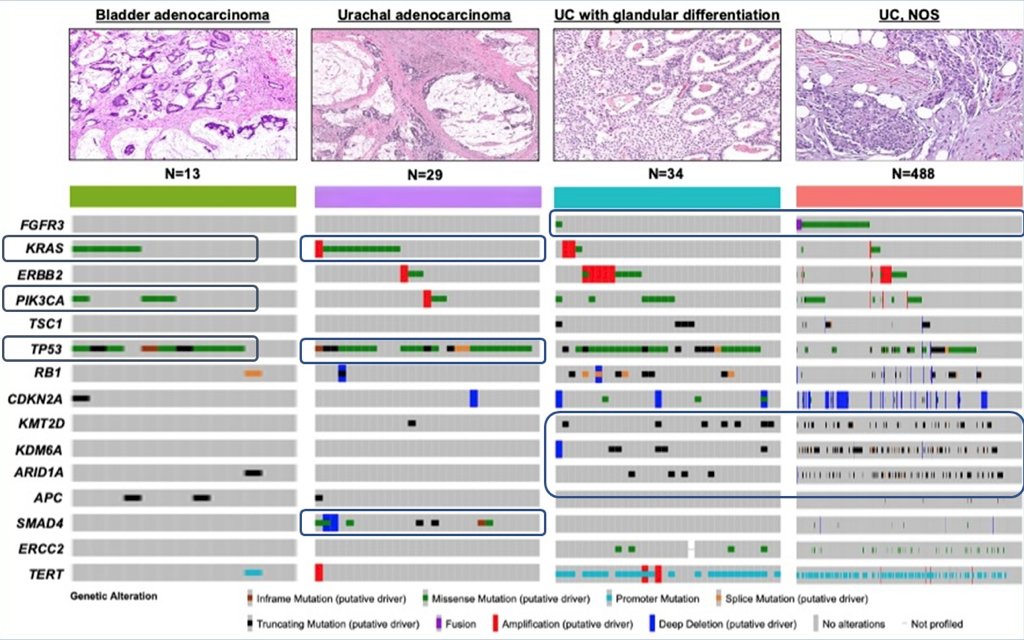
Systemic therapy for bladder adenocarcinomas should include regimens used for treating conventional urothelial carcinoma, particularly when there is glandular differentiation. However, the optimal therapy for pure bladder adenocarcinoma and urachal adenocarcinomas remains to be defined. 5-FU based regimens are the most commonly used, and the similarity to colon cancer has led to wide use of FOLFOX chemotherapy and consideration of using biologics such as bevacizumab or EGFR inhibition based on the KRAS mutation status. Ifosfamide/paclitaxel/cisplatin has activity in bladder adenocarcinoma, and the regimen of 5-FU-cisplatin-gemcitabine-leucovorin has been reported to show promise.
Plasmacytoid urothelial carcinoma is characterized by E-cadherin loss (CDH1 mutation, methylation, or other loss expression). This histologic variant has a high propensity for locoregional spread of disease via direct extension, with the extent of disease typically greater than the clinical stage indicates. Upstaging at surgery is common, even after preoperative therapy, with high rates of positive surgical margins. Outcomes are inferior to urothelial carcinoma, but likely driven by occult early spread, but with similar outcomes when controlled for stage. SEER data suggests perioperative therapy is not helpful but there are many confounders in the data. Single agent immunotherapy appears to be active, with ORR reported to be 32% in retrospective series.
Schistosomal bladder cancer is generally treated with gemcitabine and platinum chemotherapy for metastatic disease, with phase II trials of gemcitabine/cisplatin reporting objective response rates of 55%. For this disease variant, the impact of neoadjuvant chemotherapy is unclear. Conversely, non-schistosomal bladder cancer is generally chemoresistant, with 5-FU based regimens, taxanes, and other cytotoxic therapies being generally used with limited effect.
Immunotherapy for variant histology tumors has limited prospective data. In the SAUL study1 assessing atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma, 47 patients had an ORR of 9%. Durvalumab + tremelimumab had no responses for 13 patients, and ipilimumab + nivolumab had an ORR of 37% in 19 patients:

The ALLIANCE A031702 ICONIC study is assessing ipilimumab, cabozantinib, and nivolumab in rare genitourinary cancers:

Dr. Rosenberg concluded his presentation of systemic therapy for variant histology urothelial carcinoma with the following take-home messages:
- Systemic therapy for variant histology tumors remains suboptimal
- Tumors with divergent differentiation/mixed histology are generally treated with similar to conventional urothelial carcinoma
- Small cell cancer, regardless of platinum eligibility, benefit from neoadjuvant therapy, with treatment mirroring small lung cancer guidelines
- Plasmacytoid and micropapillary tumors tend to spread early, whereas adenocarcinomas are non-schistosomal squamous cell cancers and relatively chemoresistant
- Immune checkpoint blockade as later lines of therapy may have activity, with early data assessing nivolumab + ipilimumab showing promise
- Clinical trials enrolment is preferable when available
Presented By: Jonathan E. Rosenberg, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter uring the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol 2019 Jul;76(1):73-81.


