(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Dr. Tagawa presented in a session highlighting novel therapies in bladder cancer and their toxicities. Building on Dr. Tolcher’s lecture describing the basic principles of tumor antigen targeting with antibody-drug conjugates (ADCs), Dr. Tagawa provided a clinical perspective on the use of ADCs in advanced urothelial carcinoma (aUC).
Dr. Tagawa began with a discussion of enfortumab vedotin (EV), an ADC comprising an anti-Nectin-4 monoclonal antibody conjugated to monomethyl auristatin-E (MMAE) using a protease-cleavable linker. Nectin-4 is a transmembrane adhesion molecular which is importantly expressed in 83% of urothelial carcinomas. However, it is also expressed on skin, urothelium, salivary gland ducts, esophagus, breast, and stomach tissue.
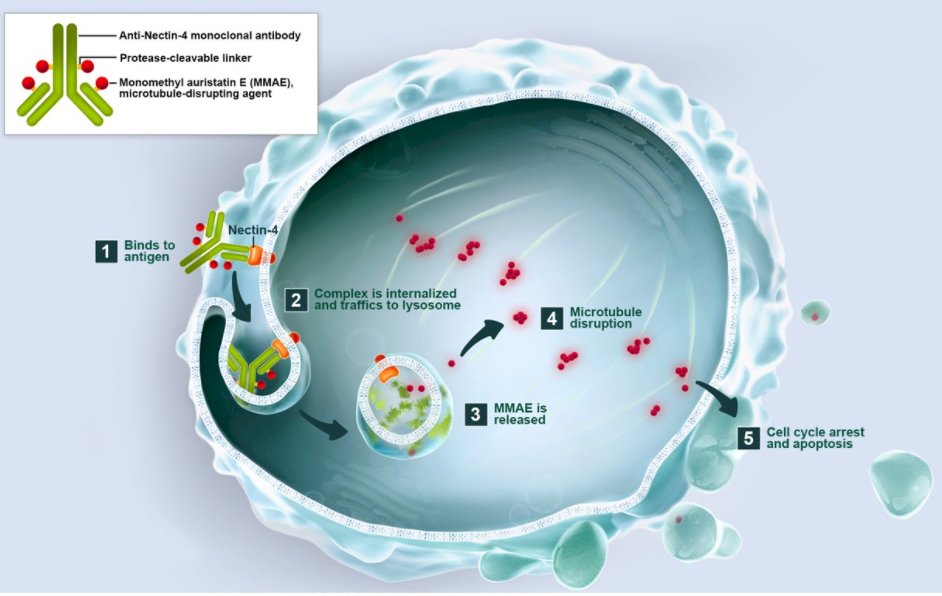
In keeping with the mechanism of action of ADCs highlighted by Dr. Tolcher, treatment with EV results in binding to Nectin-4 on urothelial cancer cells, internalization of EV followed by lysosomal trafficking and degradation. As a result MMAE is release which leads to microtubular disruption and cell cycle arrest.
The pivotal data supporting the use of EV in urothelial carcinoma came from EV-201, a dual cohort, non-randomized trial performed among patients with locally advanced or metastatic urothelial carcinoma which had prior PD-1/PD-L1 inhibitor and platinum-based chemotherapy (cohort 1; third line space) or had prior PD-1/PD-L1 inhibitor but were cisplatin ineligible (cohort 2; second line space).
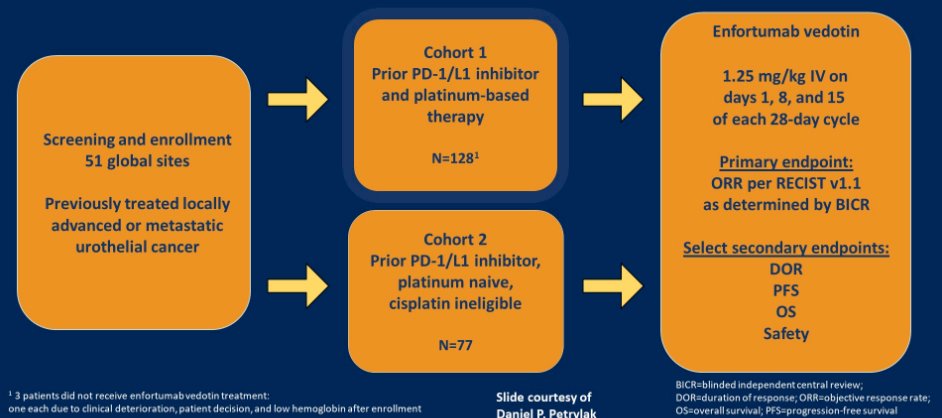
EV-201 demonstrated meaningful changes in tumor size based on blinded independent central review with an objective response rate of 44% (95% CI 35-52%) in cohort 1 and 52% (95% CI 41-62%) in cohort 2. Further long-term follow-up presented at ESMO 2020 demonstrated no surprising changes in either efficacy or tolerability.
Building on the non-randomized data from EV-201, EV-301 is a randomized, open-label phase 3 trial comparing EV to preselected chemotherapy among patients with aUC who have previously received platinum-based chemotherapy.
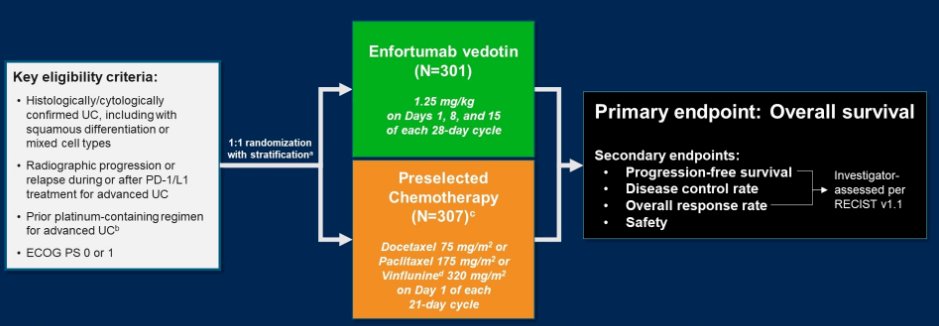
Assessing the primary endpoint of overall survival, EV showed a significantly longer overall survival (12.9 months) compared to chemotherapy (9.0 months), with a hazard ratio for death of 0.70 (95% CI 0.56-0.89). Further, progression-free survival (median 5.6 vs 3.7 months; hazard ratio 0.63, 95% CI 0.51-0.75), overall response rate s (41 vs 18%), complete response rate (5 vs 3%), and disease control rate (72 vs 53%) were significantly higher among patients who received EV. Further, these results were robust across subgroup analyses.
In terms of toxicity, EV was associated with much higher rates of rash (44 vs 10%) with grade 3 or 4 events in 14.5% of patients and dose reductions or interruptions required in 4.4% each. Further, neuropathy (46 vs 31%) and hyperglycemia (6.4 vs 0.3%) were more common among patients receiving EV. However, neutropenia and anemia were more common in patients receiving chemotherapy.
Subsequently, EV-103 was designed as a dose-escalation and dose-expansion trial aimed at assessing the role of EV in combination with a number of other agents utilized in aUC, including pembrolizumab, cisplatin, carboplatin, gemcitabine, and triplet combinations.
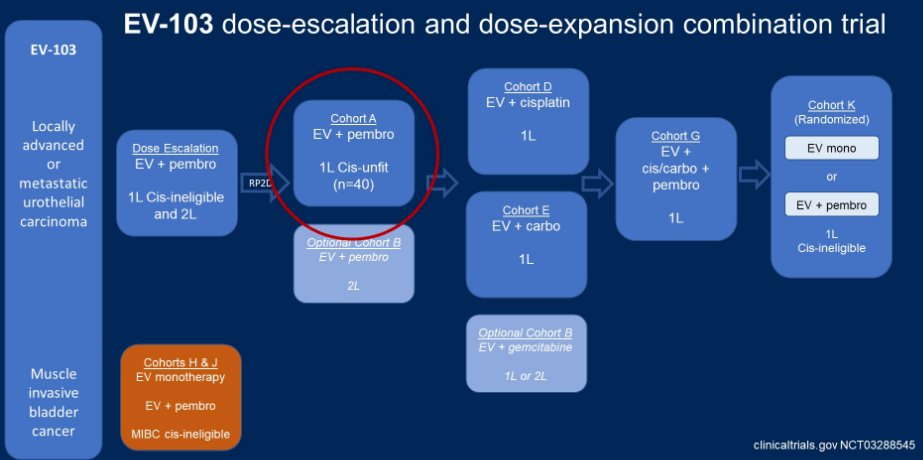
EV-103 cohort A assess the combination of EV and pembrolizumab as first-line therapy for patients with mUC who were cisplatin-ineligible. A remarkable 93% of the 43 enrolled had tumor reductions and 73% (95% CI 58-85%) had a confirmed objective response. More notably, 7 patients (16%) had complete responses. As of February 2020, EV was granted a break-through designation.
Dr. Tagawa then moved to discuss Sacituzumab Govetican (SG), an ADC comprising a humanized anti-Trop-2 antibodies linked to an SN-38 payload by a hydrolyzable linker. There are a number of features that make SG unique and appealing. Trop-2 is an epithelial cell surface antigen that is highly expressed in UC. Further, the SN-38 payload is more potent than the parent compound, irinotecan. Finally, the linker allows a high (7.6:1) drug to antibody ratio.
The first data for SG that Dr. Tagawa highlighted comes from Study 01, a phase I/II basket trial of patients with advanced epithelial tumors, including patients with urothelial disease. Following an initial phase I dose escalation phase, the phase 2 cohort expansion focused heavily on patients with mUC (n=45). These patients were heavily pre-treated. In spite of this, the objective response rate was 39% among those with 2 or fewer prior lines of therapy and 18% among those with 3 or more prior lines of therapy.
These data formed the basis of the TROPHY-U-01 study which includes five different cohorts of patients with advanced UC. Dr. Tagawa focused first on cohort 1 which included patients with mUC who had progressed on prior platinum-based chemotherapy and immune checkpoint inhibitor therapy. Among 113 patients in this cohort (of whom 50% had received 3 or more prior lines of therapy), the objective response rate was 27% (95% CI 20-37%) and 6 patients (5%) had complete response. Median progression-free and overall survivals were 5.4 and 10.9 months, respectively.
In terms of toxicity, neutropenia was seen in 46% of which grade 3 or 4 events were seen in 34%. Diarrhea was noted in 65% of patients and of grade 3 or 4 severity in 10%. 7 patients (6%) discontinued therapy as a result of adverse events and there was one treatment-related death due to febrile neutropenia.
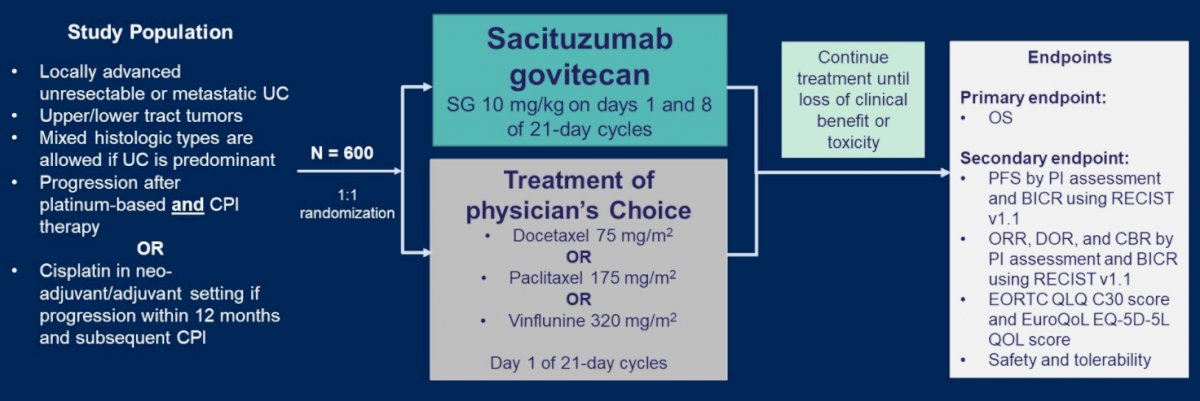
These data formed the basis of the phase III TROPiCS-04 study, a randomized comparison of SC and physician’s choice chemotherapy among patients who have progression following platinum-based chemotherapy and immune checkpoint inhibitors.
Finally, Dr. Tagawa closed by briefly mentioning RC48 (disitamab vedotin), an ADC for patients with HER2+ aUC. RC48 is an antibody drug conjugate of an anti-HER-2 antibody and MMAE which has demonstrated promising results in early data. Among 43 biomarker-selected patients, confirmed ORR was 51%, and even higher (60%) among those both HER-2 IHC and FISH positivity. Toxicity with RC48 includes neuropathy in 61% (including grade 3 in 23% and leading to discontinuation in 16%), as well as fatigue (44%), neutropenia (42% including 14% grade 3), and transaminitis (33%). RC48 was granted FDA breakthrough designation as of September 2020 and there are interesting early data combining it with PD-1 inhibitors.
In closing, Dr. Tagawa also mentioned other ADC being investigated, including oportuzumab monatox, an anti-EpCAM ADC being investigated in non-muscle invasive disease.
Presented by: Scott T. Tagawa, FACP, MD, MS, Weill Cornell Medicine


