(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma session highlighting work from Dr. Xinan Sheng and colleagues presenting two-year survival results of the POLARIS-03 study assessing toripalimab in patients with metastatic urothelial carcinoma. Patients with advanced metastatic urothelial carcinoma who experience disease progression after standard therapy have limited treatment options. Toripalimab was approved in China (April 2021) for the 2nd line treatment of metastatic urothelial carcinoma based on a phase II clinical study (POLARIS-03) in Chinese patients with metastatic urothelial carcinoma. At GU ASCO 2022, Dr. Sheng and investigators report the two-year OS update and biomarker analysis of the study.
Metastatic urothelial carcinoma patients received toripalimab 3 mg/kg Q2W until disease progression, unacceptable toxicity, or voluntary withdrawal. Clinical response was assessed every 8 weeks. The primary endpoint was ORR, and tumor PD-L1 expression, tumor mutational burden (TMB), and other biomarkers were also evaluated for correlation with clinical response. Secondary endpoints included PFS and OS.
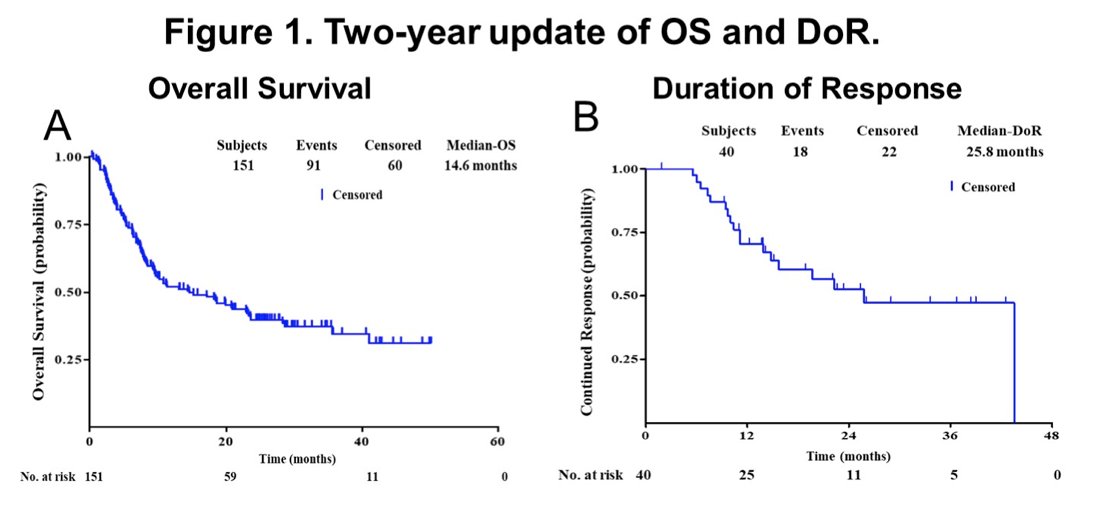
From May 2017 to September 2019, 204 patients were screened and 151 patients were enrolled from 15 participating centers. By the cutoff date of September 8, 2021, no emergent new safety signal was identified compared with the previous one-year report. By the cutoff date, 91 patients have died, and the median OS was 14.6 months. In the ITT population, the ORR was 26.5% (95% CI 19.7-34.3) and disease control rate was 45.0% (95% CI 36.9-53.3). The median time to response was 1.8 months (95% CI 1.7-1.9), median PFS was 2.3 months (95% CI 1.8-3.6), median OS was 14.6 months (95% CI 9.3-23.3), and median duration of response was 25.8 months (95% CI 13.9-43.5):
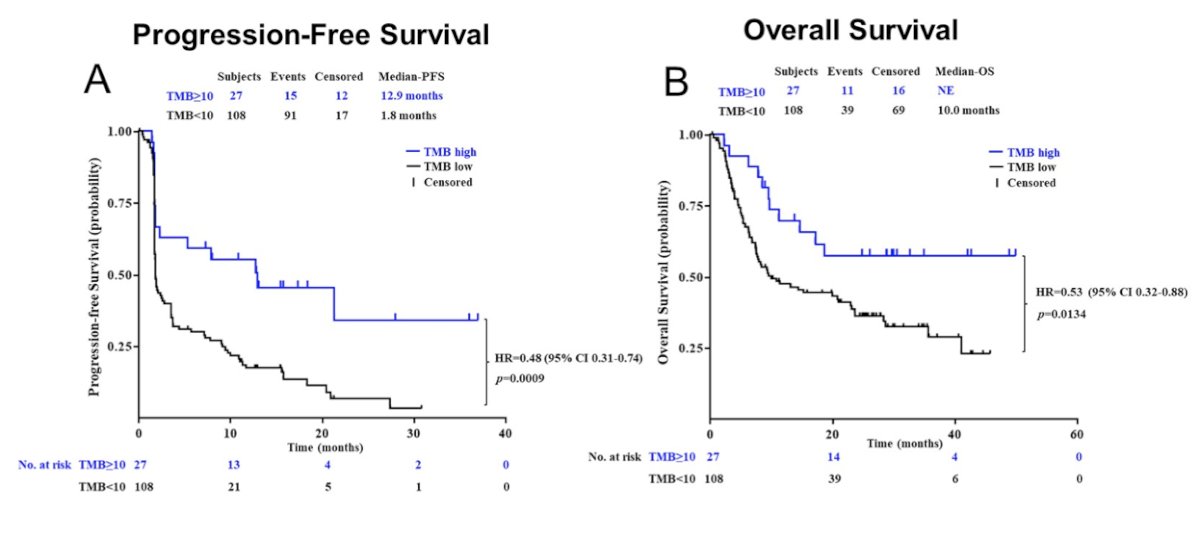
Whole exome sequencing (WES) was performed on tumor biopsies and paired PBMCs and the results were available from 135 patients. Patients with mutations in chromatin remodelers SMARCA4/PBRM1 or tumor suppressor RB1 were associated with better responses to toripalimab than patients with wild-type genes. The ORR was 30% (6/20) in patients with FGFR2/FGFR3 mutations or FGFR2/FGFR3 gene fusions, and 42% (5/12) in patients with NECTIN4 genomic alternations. The median TMB value was 4.1 mutations per million base pairs (Mb) in the cohort. Using 10 mutations/Mb as the cut off, TMB-high patients (n = 27) had better ORR than TMB low patients (n = 108) (48% versus 22%, p = 0.014). The TMB-high group also showed better PFS (12.9 versus 1.8 months; HR 0.48, 95% CI 0.31-0.74) and OS (not reached versus 10.0 months; HR 0.53, 95% CI 0.32-0.88) than the TMB low group:
Dr. Sheng concluded this presentation of updated results from the POLARIS-03 of toripalimab with the following take-home messages:
- Toripalimab has demonstrated a manageable safety profile and encouraging clinical activity in metastatic urothelial carcinoma patients refractory to 1st line chemotherapy
- WES analysis identified divergent mutations in the study
- TMB has the utility to predict not only the response rate but also the PFS and OS benefits in patients with metastatic urothelial carcinoma in response to an ICI monotherapy
Clinical trial information: NCT03113266.
Presented by: Xinan Sheng, Key laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Genitourinary Oncology, Peking University Cancer Hospital & Institute, Beijing, China
Co-Authors: Haige Chen, Bin Hu, Xudong Yao, Ziling Liu, Xin Yao, Hongqian Guo, Yi Hu, Zhigang Ji, Hong Luo, Benkang Shi, Jiyan Liu, Jin WU, Fangjian Zhou, Zhisong He, Jinhai Fan, Yiran Huang, Jun Guo
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


