(UroToday.com) In a poster presentation on the third day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focussed on Renal Cell Cancer; Adrenal, Penile, Urethral, and Testicular Cancers, Dr. Ulka N. Vaishampayan presented the rationale and design of the PROBE trial, examining the role of cytoreductive nephrectomy among patients undergoing immune checkpoint inhibitor therapy for advanced kidney cancer.
Patients who present with de novo metastatic kidney cancer with synchronous primary tumor and metastases have a poorer prognosis than those who develop metachronous metastases after nephrectomy. While cytoreductive nephrectomy was, for many years, a standard of care given data from the interferon-era, the CARMENA trial demonstrated no change in overall survival with the addition of nephrectomy to sunitinib therapy and thus questioned the role of cytoreduction in patients receiving tyrosine kinase inhibitors, such as sunitinib.
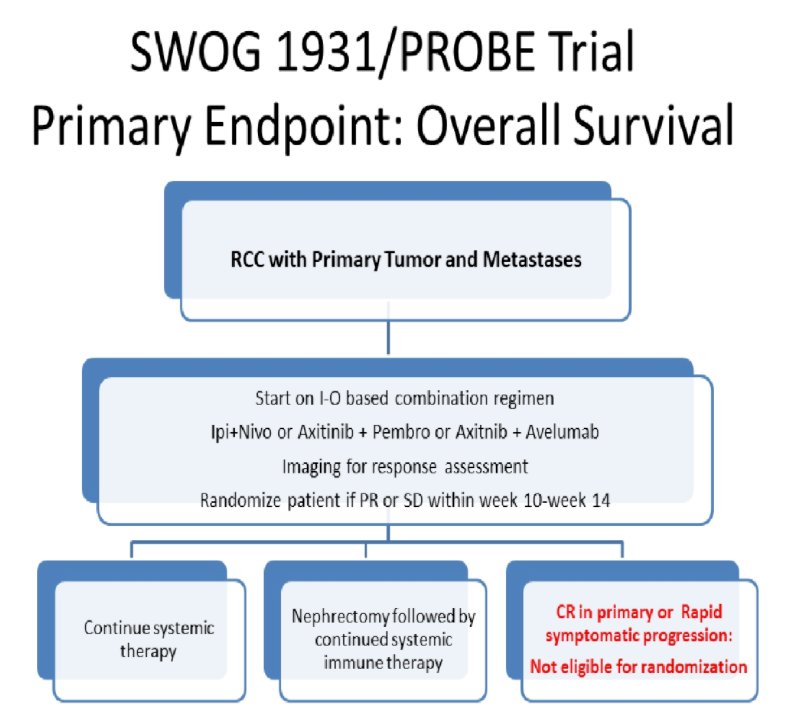
However, since the design and conduct of CARMENA, the standard first line systemic therapy in advanced renal cell carcinoma (RCC) has evolved and immune checkpoint based combination therapy has now become the standard of care. However, no trials have assessed the role of cytoreductive nephrectomy (CN) or primary resection in the setting of immune checkpoint based systemic therapy, a question the PROBE study (NCT04510597) aims to address. The underlying postulated mechanism is that the broader antigen spread and higher neoantigen load enabled by the primary tumor would enhance the efficacy of the immune therapy. CN after initial systemic therapy will potentially enable eradication of the immune resistant clones within the primary.
The authors will enroll patients with histologically proven metastatic RCC with the primary tumor in place. Patients should be treated with one of the FDA approved ICI based combinations including ipililumab and nivolumab, axitinib and pembrolizumab, or axitinib and avelumab. Cabozantinib and nivolumab and lenvatinib and pembrolizumab combinations are being added in forthcoming amendments.
Imaging must be performed in the 12 weeks prior to pre-randomization treatment. Patients must be deriving clinical benefit and deemed a surgical candidate according to the study urologist. Randomization occurs between 10-14 weeks of therapy. Notably, for patients who have already been initiated on systemic therapy, both registration and randomization can occur together.
Patients will be randomized in a 1:1 fashion to receive CN followed by continued systemic therapy or to continue on systemic therapy. Randomization will be stratified by pre-randomization immunotherapy status, Zubrod performance status, systemic agent combination, and 12 week disease response.
Patients will be followed for up to 7 years from randomization to assess the primary endpoint of overall survival.
Using an estimate of median survival of 25 months in the non-surgical arm and 37 months among those undergoing CN, the authors will need a total of 302 eligible randomization patients to demonstrate a hazard ratio of 0.68 with a .one-sided alpha = 0.025 and 85% power.
There are a number of other secondary objectives including comparing overall survival among those who received assigned treatment, progression-free survival, complications, objective response rate, and change in primary tumor diameter at week 12 assessment.
Presented by: Ulka N. Vaishampayan, MD, University of Michigan Cancer Center, Detroit, MI

