(UroToday.com) The 2022 GU ASCO Annual meeting included a rare tumors oral abstract session featuring work from Dr. Hielke Martijn de Vries and colleagues presenting results of PERICLES, a phase II trial investigating atezolizumab +/- radiotherapy for advanced squamous cell carcinoma of the penis. Patients with advanced squamous cell carcinoma of the penis have a poor prognosis (21% 2-year overall survival from the moment of diagnosis) and high morbidity, due to progressive locoregional disease. Pre-clinical studies show high rates of infiltrating immune cells (high number of intratumoral CD8 T-cells) and high PD-L1 expression (40-60%), suggesting that immunotherapy may be beneficial for these patients:
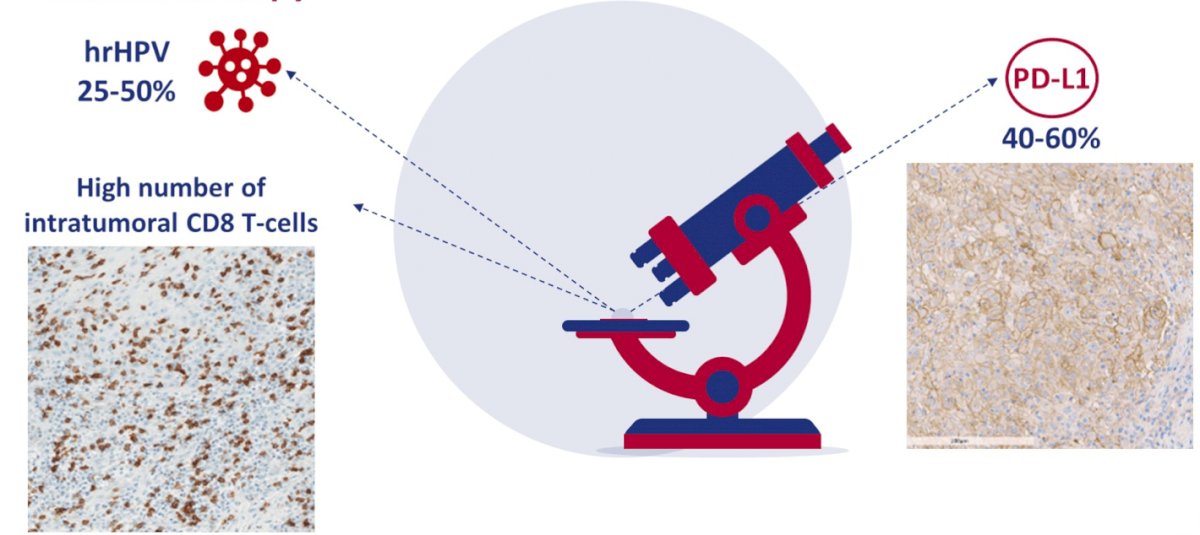
In the PERICLES study, the investigators assessed the activity of atezolizumab in advanced squamous cell carcinoma of the penis patients, with or without radiotherapy to control locoregional lymph node disease.
This was a single-centre phase 2 study with two treatment arms (non-randomized) and was conducted in 32 histologically confirmed advanced squamous cell carcinoma of the penis patients with a WHO performance status of 0-1 (NCT03686332). Any previous treatment except for immunotherapy was allowed and study treatment consisted of atezolizumab 1200 mg every 3 weeks (all patients). Patients expected to benefit from radiotherapy for locoregional disease control (cohort A) additionally received 33 fractions of 1.5 Gy (locoregional affected lymph node regions and penile region) and 1.8 Gy (macroscopic tumor + margin) irradiation. Response was evaluated with 12-weekly CT scans of the abdomen and thorax using RECIST1.1. Toxicity was scored by NCI-CTCAE V4. The primary endpoint was 1-year progression free survival for the full cohort. The PERICLES trial design is as follows:

From October 2018 to August 2021, 20 patients were included in cohort A (radiotherapy + atezolizumab) and 12 patients in cohort B (only atezolizumab). Median follow-up was 22 months (IQR 4.3-14). The median age was 67 years (IQR 60-72) and all patients had stage IV advanced squamous cell carcinoma of the penis. Patients received:
- Surgery (70%)
- Prior radiotherapy (34%)
- No prior treatment (25%)
- Chemoradiation (22%)
- Chemotherapy (6%)
An immunotherapy or radiotherapy-related grade 3-4 adverse event was observed in 3/32 (9.4%) and 1/20 (5.0%) patients, respectively, and there were no grade 5 treatment-related adverse events. One-year progression free survival was 12.5% (95% CI 5.0%-31.3%), which did not meet the primary objective:

The response rate of RECIST1.1 evaluable patients was 33%:
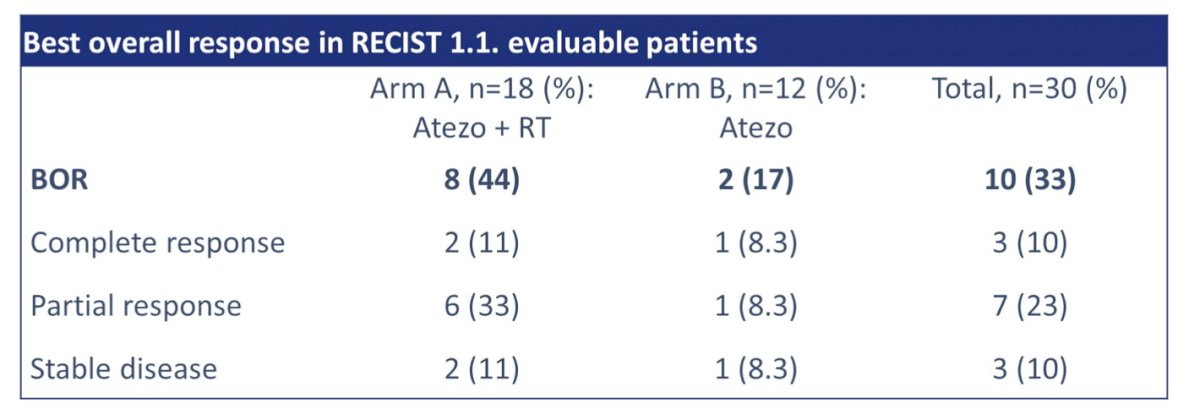
In two patients with pulmonary metastases, a complete response was observed, and initial responses with early progression were seen in five patients. The median overall survival in this cohort was 11.5 months (95% CI 5.4-19.7):

Dr. de Vries concluded this presentation of PERICLES, a phase II trial investigating atezolizumab +/- radiotherapy for advanced squamous cell carcinoma of the penis, with the following take-home messages:
- Anti-tumor activity of atezolizumab was observed in advanced squamous cell carcinoma of the penis, including complete and durable responses
- The trial failed to meet its primary objective (progression free survival)
- There was no evidence to suggest a synergist effect of radiotherapy – was this secondary to a suboptimal radiotherapy schedule?
- These results suggest that a subset of advanced squamous cell carcinoma of the penis patients has incomplete immunological activity and/or early resistance to atezolizumab – analysis of tumor tissue (including on-treatment biopsies) is ongoing. Perhaps combination therapies are necessary to overcome resistance
- Analysis of tumor tissue collected in this trial (including on-treatment biopsies) could suggest new therapeutic strategies to overcome resistance and improve clinical outcome to immunotherapy in advanced squamous cell carcinoma of the penis
Clinical trial information: NCT03686332.
Presented by: Hielke Martijn de Vries, Netherlands Cancer Institute, Amsterdam, Netherlands
Co-Authors: Jeantine De Feijter, Elise Bekers, Marta Lopez-Yurda, Floris J. Pos, Simon Horenblas, Ekatarina S. Jordanova, Oscar R. Brouwer, Eva E. Schaake, Michiel Simon Van Der Heijden
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


