This is a three-part, open-label, parallel-cohort safety and tolerability study of relugolix in combination with:
- Part 1: Abiraterone acetate in men with metastatic castration-sensitive prostate cancer (mCSPC) or metastatic castration-resistant prostate cancer (mCRPC)
- Part 2: Apalutamide in men with mCSPC or non-metastatic castration-resistant prostate cancer (nmCRPC)
- Part 3: Docetaxel in men with mCSPC or mCRPC
The full study design is as follows:
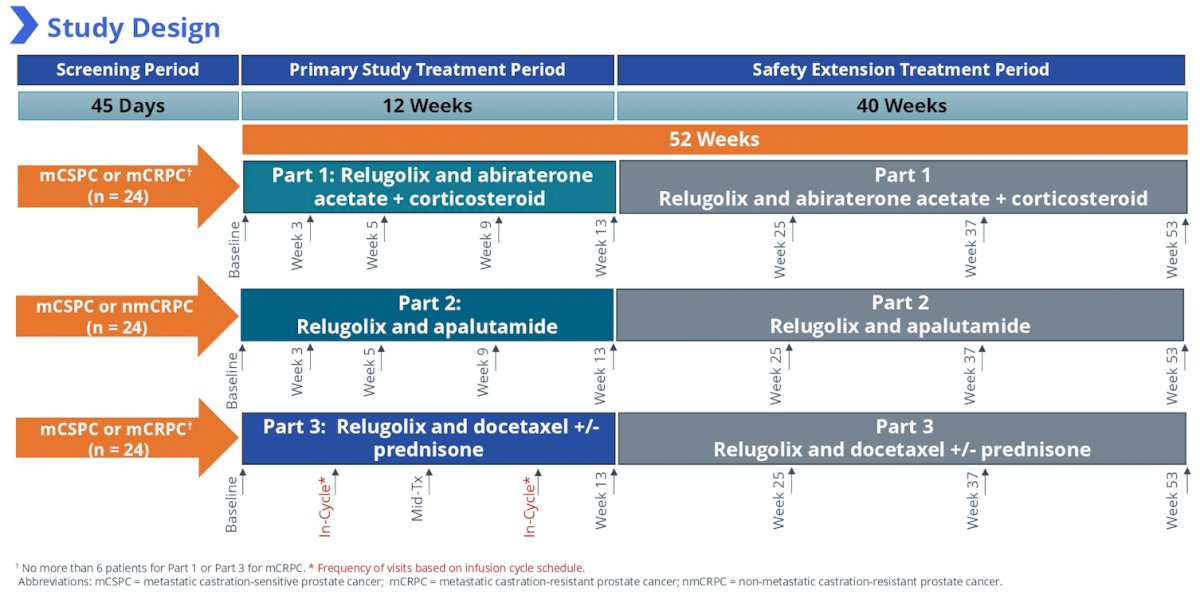
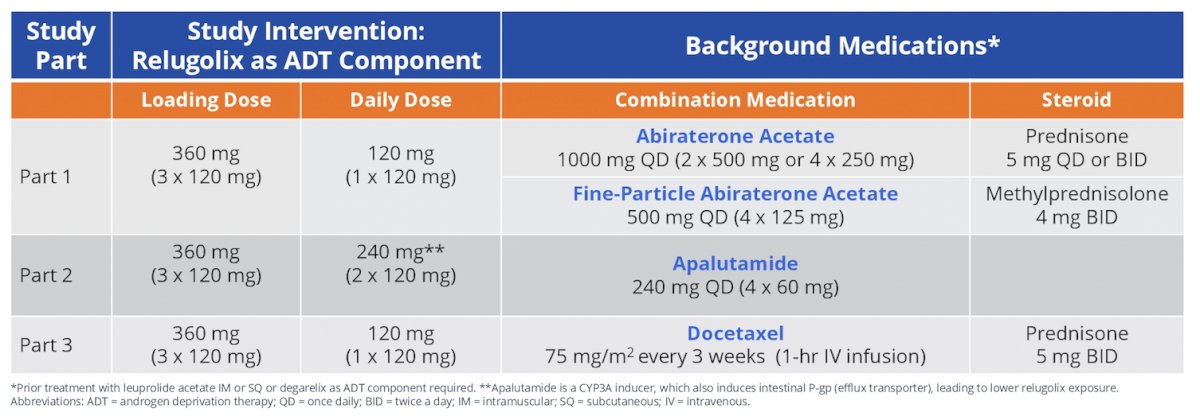
Each part of the study consists of a 45-day screening period, a 12-week primary study treatment period, and a 40-week safety extension treatment period. All of the men are required to have been treated with leuprolide acetate or a GnRH receptor antagonist (eg. degarelix) in combination with abiraterone acetate for a minimum of 12 weeks, apalutamide for a minimum of 6 weeks, or docetaxel for a minimum of one treatment cycle prior to the baseline (Day 1) visit. Men will be transitioned from leuprolide acetate or degarelix to relugolix (120 mg [Part 1 and 3] or 240 mg [Part 2] once daily after a single loading dose of 360 mg). On the approximate date the next analog or antagonist injection is scheduled, treatment with each combination treatment will continue as previously prescribed.
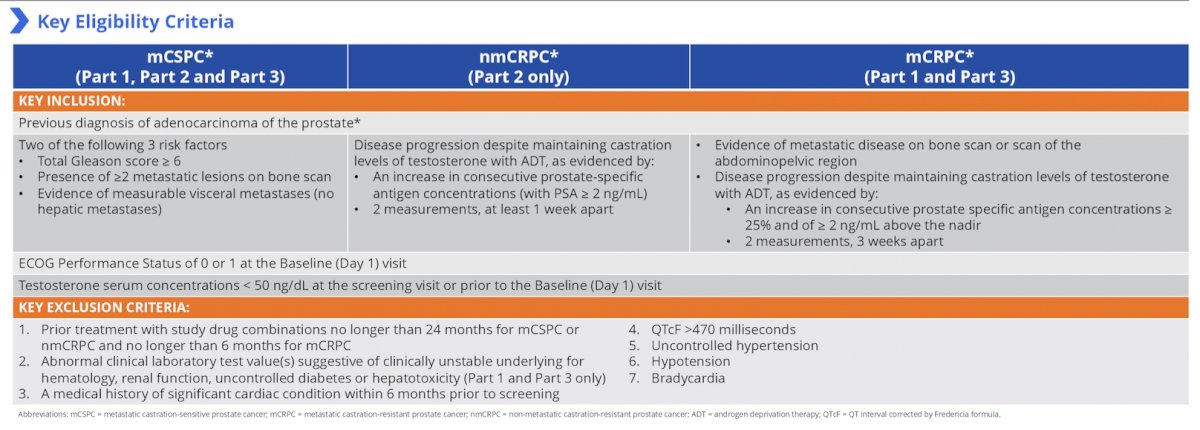
Key eligibility criteria, stratified by mCSPC, nmCRPC, and mCRPC are as follows:
As such, the study will provide safety and tolerability of relugolix and the three different combination agents for up to 1 year and in addition, will provide safety and tolerability data as men transition from injectable leuprolide acetate or degarelix to oral treatment with relugolix. The primary outcome measures are safety parameters, including: clinical laboratory tests, vital sign measurements, ECG parameters, and adverse events. Additional assessments include testosterone and PSA concentrations and drug concentrations during the 12 week primary study treatment period only. Enrollment into the study began in March 2021, and a protocol amendment was approved in July 2021 to include Part 2 and Part 3 of the study, as well as to add the 40-week safety extension treatment period. Screening for Part 2 was initiated in August 2021 and for Part 3 it is expected to initiate in February 2022.
Clinical trial information: NCT04666129.
Presented by: Jose De La Cerda, MD, Urology San Antonio, San Antonio, TX
Co-Authors: Elizabeth Migoya, Bruce Brown, Sophia Lu, Fabian Zohren, Ronald F. Tutrone, Curtis Dunshee
Affiliations: Urology San Antonio, San Antonio, TX, Myovant Sciences, Inc., Brisbane, CA, Pfizer Inc., New York, NY, Chesapeake Urology, Towson, MD, Urological Associates of Southern Arizona, Tucson, AZ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 17 – Sat, Feb 19, 2022.
References:


