(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. Gonzalez presented work on patient-reported quality of life metrics among men receiving prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) for the treatment of metastatic castration-resistant prostate cancer (mCRPC). PSMA-RLT conjugates a radionuclide to a small molecule ligand with affinity for PSMA. PSMA-RLT utilizing Lutetium-617 has recently been demonstrated to improve progression-free survival, overall survival, and patient-reported outcomes (PROs) in the TheraP and VISION trials. However, there is a relative dearth of knowledge about the effects of PSMA-RLT on symptoms related to bone metastasis (BM), a common cause of significant morbidity in men with mCRPC.
Between 2015 to 2017, the authors treated 50 patients with up to 4 cycles of 177Lu-PSMA-617 every 6 weeks (trial registration: ACTRN12615000912583). Patients completed a validated assessment of bone metastasis-related pain (EORTC QLQ-BM22) prior to each cycle and 6 or 12 weeks after the last cycle of 177Lu-PSMA. The authors then assessed the change in pain over the course of the study using linear mixed effects models and examined whether unadjusted changes in PROs exceeded published cut-offs for minimal clinically important differences.
They noted that patients reported reduced bone metastasis-related pain over time (p =.01) when receiving PSMA-RLT. Notably, among 44 patients completing the EORTC QLQ-BM22 before cycle 2, 22 (50%) reported clinically significant improvement in pain.
Over longer follow-up, among 37 patients alive and evaluable, 25 provided evaluable data on bone metastasis-related pain. Of these, 10 (40%) reported clinically significant improvement in pain following treatment. Patients also reported less functional interference from pain over time (p <.01) and lower consistency, intermittency, and difficulty alleviating pain with medication over time (p =.01).
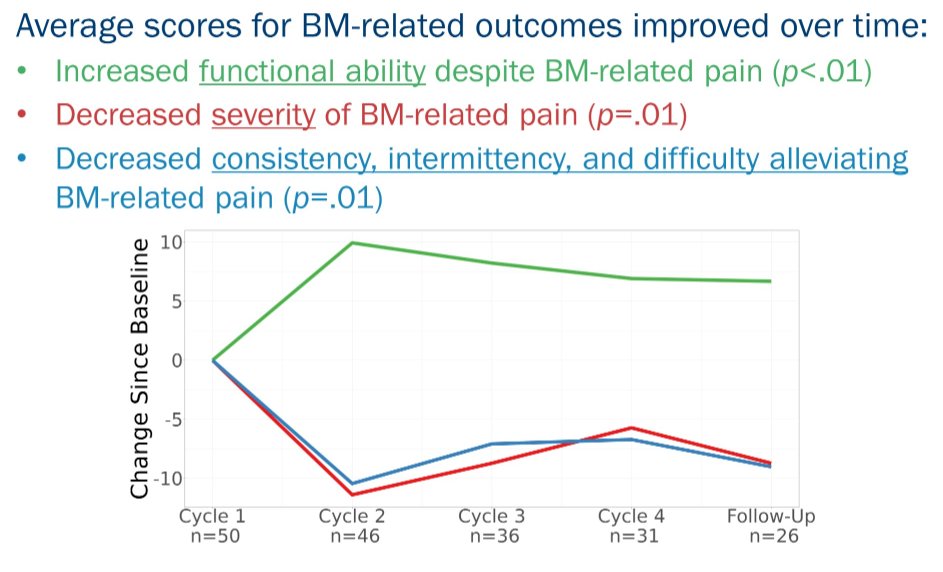
Both before cycle 2 and following their last cycle of PSMA-RLT, 26% and 38% of respondents reported a clinically significant reduction in functional interference from pain. At the same time points, 33% and 42% of respondents reported clinically significant improvement in consistency, intermittency, and difficulty alleviating pain with medication.
Thus, Dr. Gonzalez concluded that PSMA-RLT with 177Lu-PSMA resulted in improvements in bone metastasis-related symptoms. At follow-up, 38-42% of patients completing PRO assessment reported clinically meaningful improvements in bone metastasis-related pain, a significant contributor to quality of life. Clinical trial information: ACTRN12615000912583.
Presented by: Brian Gonzalez PhD, Moffitt Cancer Center, Tampa, FL

