(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. Lorente presented an analysis of patients experiencing PSA-only progression among men enrolled on the LATITUDE trial (NCT01715285) of abiraterone acetate in conjunction with androgen deprivation therapy (ADT) for men with high-risk metastatic hormone-sensitive prostate cancer (mHSPC). The LATITUDE trial, along with data from STAMPEDE, demonstrated the survival benefit of abiraterone acetate in this population and formed the basis for its approval.
Notably, by Prostate Cancer Working Group 3 (PCWG3) guidelines, patients who have evidence of PSA progression in the absence of clinical or radiographic progression should not discontinue therapy. However, the frequency of this clinical scenario, and its prognostic significance, is unclear.
Thus, the authors performed a retrospective, post-hoc analysis of patients treated in the LATITUDE trial (YODA project #2020-4298). Using PCWG2 criteria, the authors identified confirmed PSA progression and radiographic progression. Those patients who had PSA progression and radiologic evaluation demonstrating the absence of PSA progression were defined as having PSA-only progression.
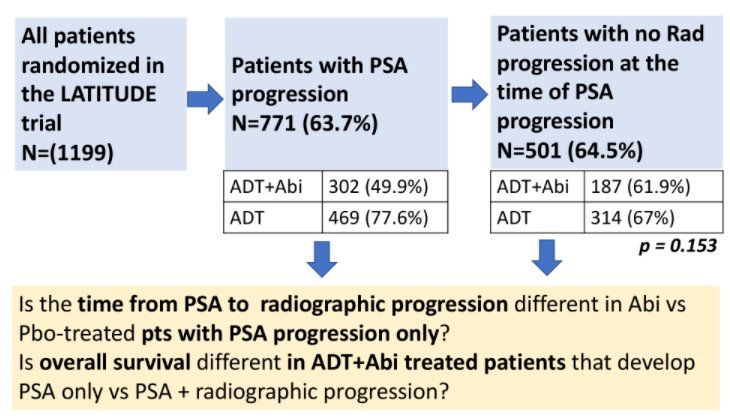
In these patients, time from PSA to radiographic progression was defined as the time (months) from the date of confirmed PSA progression to the date of radiographic progression. The Kaplan-Meier technique and Cox proportional hazards models were used to define median survival times and to compare outcomes of men treated with abiraterone acetate versus placebo.
In patients enrolled on LATITUDE, 771 men (63.7%) experienced PSA progression, with higher rates seen among those men receiving placebo (469 patients, 77.6%) compared to those receiving abiraterone acetate (302 patients, 49.9%). Among these 771 men, 501 (64.5%) had no evidence of radiographic progression on the coinciding radiographic evaluation and were thus defined as having PSA-only progression. The proportion of PSA-only progression did not significantly differ between those patients receiving abiraterone acetate (n=187, 61.9%) and those receiving placebo (n=314, 67%) (p = 0.153).
The authors found that 207 (41.3%) patients had measurable disease at baseline, comparable to the overall study population (43.8%). Among the 501 men with PSA-only progression, 315 (62.9%) subsequently showed evidence of radiographic progression. Of these, subsequent radiographic progression was seen in 105 men (56.1%) receiving abiraterone acetate and 210 (66.9%) receiving placebo (p = 0.017).
The median time from PSA-only progression to radiographic progression was 8.4 months (95%CI: 8.1-9.2), with any significant differences between the two treatment arms (9.2 vs 8.3 months; HR: 0.88 [95%CI: 0.7-1.1]; p = 0.287).
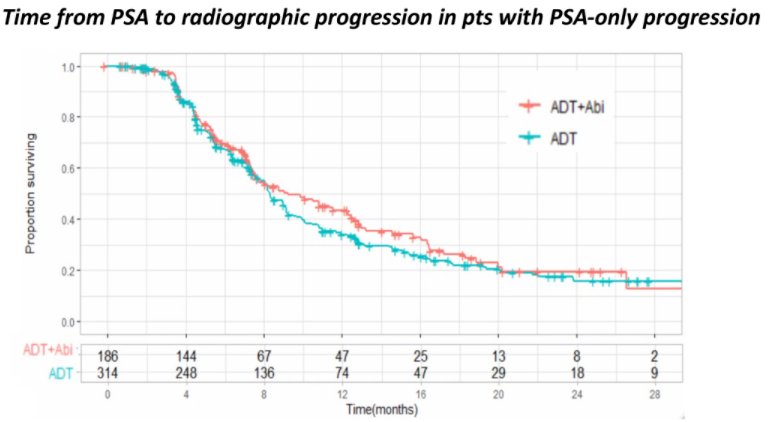
Among patients who received abiraterone acetate, the median overall survival from the time of PSA progression was 19.8 months (95%CI:17.2-24). This time was longer for patients who had PSA-only progression than those who had simultaneous PSA and radiographic progression (24 vs 15.3 months; HR: 0.62; p = 0.007).
Thus, the authors concluded that adequately designed trials are needed to assess the benefit of continuing AR signaling inhibitors in patients who have PSA progression. These data highlight the importance of both PSA and radiographic follow-up for patients with mHSPC.
Presented by: David Lorente MD, Medical Oncology Department, Hospital Provincial de Castellón

