(UroToday.com) On the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Poster Session A focussed on the care of patients with prostate cancer. Dr. provided a poster presentation of the updated overall survival results of the ProCAID trial which examined capivasertib with docetaxel versus docetaxel alone in men with metastatic castration resistant prostate cancer (mCRPC). Capivasertib is a potent selective inhibitor of all three AKT isoforms (AKT1/2/3), a pathway which is frequently deregulated in mCRPC. The primary report of ProCAID should no difference in composite progression free survival (cPFS), the primary outcome. However, overall survival, a key secondary endpoint, was extended in the capivasertib plus docetaxel arm, with consistent results independent of biomarker status.
In this report, the authors provided updated analysis of mature OS data, once ≥65% events had been identified. To briefly recap, ProCAID is a randomized, double blind, placebo controlled phase II study which compared capivasertib with docetaxel versus docetaxel with placebo. Within the intention to treat population (n=150), the authors used Cox proportional hazards model, adjusted for minimisation factors to assess the effect of capivasertib with docetaxel on overall survival. Additionally, they performed subgroup analyses defined based on prior androgen receptor targeted agent (ARTA) exposure to abiraterone and/or enzalutamide and the balance of post-trial life extending treatment use by treatment arm.
At the time of data cut-off, 99 patients (66.0%) had died, with 88 (88.9%) attributable to prostate cancer. Only 5 patients (3.3%) remained on capivasertib or placebo. The median overall survival was 25.3 months for the capivasertib plus docetaxel arm vs. 20.3 months for placebo plus docetaxel (hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.47 to 1.05; p = 0.09).

Notably, most patients in both arms (n=99, 66%) received one, or more, subsequent life extending treatment options, including abiraterone, enzalutamide, radium-223 and cabazitaxel, were, with relatively similar treatment allocation between the two groups.
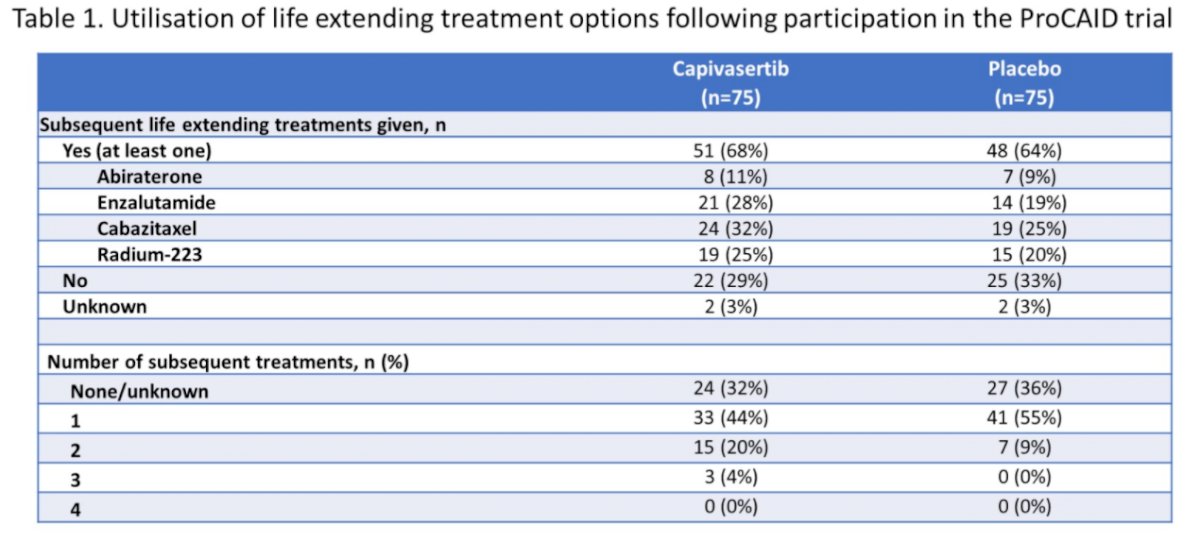
Just over two-thirds of the cohort (n=101) received abiraterone or enzalutamide prior to enrollment. Among these patients, there was a statistically significant benefit to the combination of capivasertib with docetaxel (median OS 31.1 months) compared to docetaxel and placebo (median OS 19.3 months; HR 0.57, 95% CI 0.36 to 0.91). In contrast, among patients who had not received abiraterone or enzalutamide, there was no benefit to the combination approach.
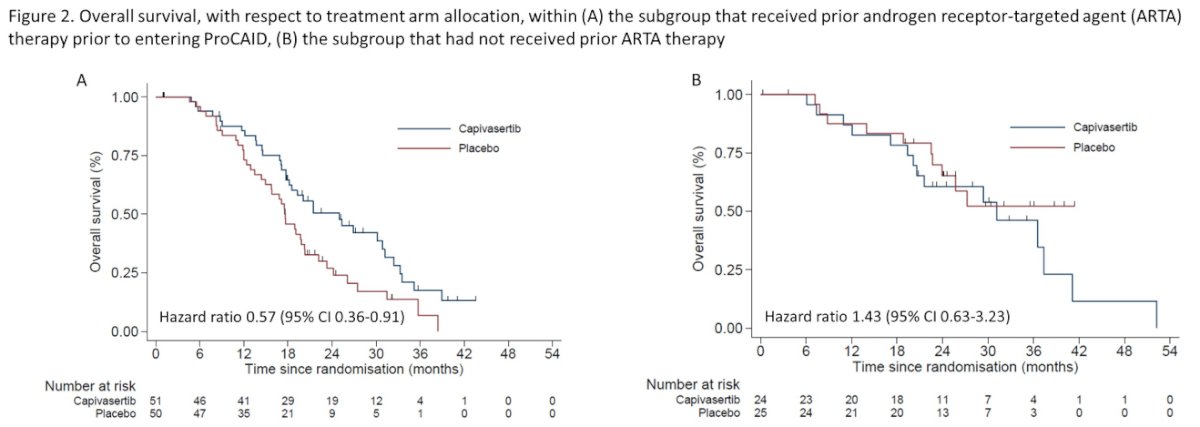
101 pts (67.3%; 51 capivasertib, 50 placebo) had received abi/enza prior to entering ProCAID. Within this subgroup, but not in the remaining 49 pts who were naive to prior abi/enza (median OS 31.1 vs. not reached respectively; HR 1.43, 95% CI 0.63 to 3.23). T
Notably, there was no evidence of effect medication by biomarker status for PI3K/AKT/PTEN pathway activation and safety signals were in keeping with the initial report of ProCAID.
Thus, Dr. Crabb concluded that the overall analysis of overall survival in ProCAID demonstrates a non-significant prolongation of overall survival with the addition of capivasertib to docetaxel for mCRPC. However, in the subset of men who had previously received abiraterone or enzalutamide, a significant benefit was seen, a finding which warrants prospective trials.
Presented by: Simon J. Crabb, PhD, MBBS, Southampton Clinical Trials Unit, University of Southampton, Southampton, United Kingdom


