(UroToday.com) In this presentation, Dr. Fred Saad presented results of the PROpel study. PROpel is a randomized phase III trial of abiraterone plus olaparib versus abiraterone plus placebo as first-line therapy for patients with metastatic castration-resistant prostate cancer (mCRPC).
Patients treated in the first-line mCRPC setting have a median survival of approximately three years in clinical trials. In clinical practice, only about half of these patients receive second-line therapy for mCRPC and median survival is less than two years. This highlights the major unmet need to improve outcomes in the first-line mCRPC treatment setting. A phase III study of olaparib monotherapy demonstrated significantly improved radiographic progression-free survival (rPFS) and overall survival (OS) in men with homologous recombination repair (HRR) deficient mCRPC following progression on prior androgen receptor pathway inhibitor (ARPI).[1] A phase II study of olaparib plus abiraterone improved rPFS compared to abiraterone alone in men with mCRPC irrespective of HRR mutation status.[2] PROpel is the first phase III study to evaluate the combined effect of a PARP inhibitor plus an ARPI in first-line mCRPC irrespective of HRR mutation status.
The combination of PARP inhibitor plus ARPI is based on strong scientific rationale. PARP inhibition is reported to increase activity of ARPIs through AR-dependent transcription. ARPIs are reported to induce HRR deficiency and thus increase susceptibility to PARP inhibition. The investigators hypothesized that the combined effect would result in anti-tumor activity in HRR mutant and HRR non-mutant mCRPC.
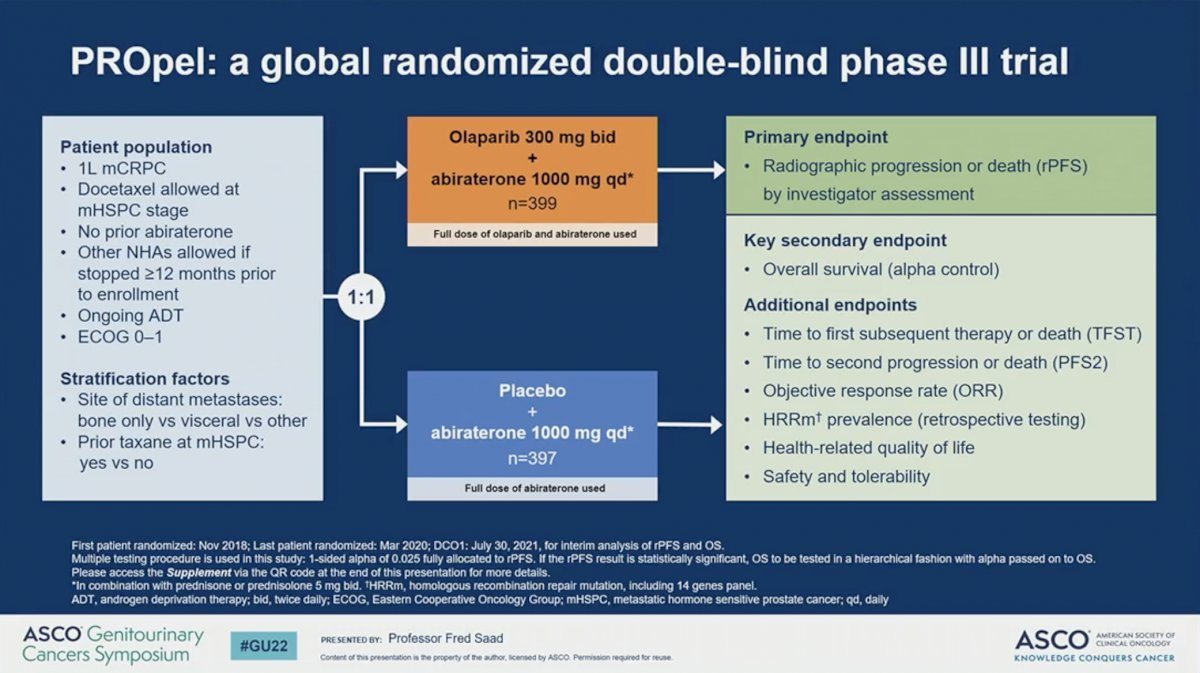
PROpel is a global, randomized, double-blind phase III trial in men with mCRPC. Eligible participants had received no prior abiraterone and had stopped an alternative ARPI more than 12 months prior to enrollment. Docetaxel for metastatic hormone-sensitive prostate cancer (mHSPC) was allowed. Patients were stratified by site of distant metastasis and whether they had received docetaxel for mHSPC. Patients were then randomized to receive full-dose abiraterone 1000 mg daily with either placebo or full-dose olaparib 300 mg BID. The primary endpoint was investigator-assessed rPFS. OS was the key secondary endpoint.
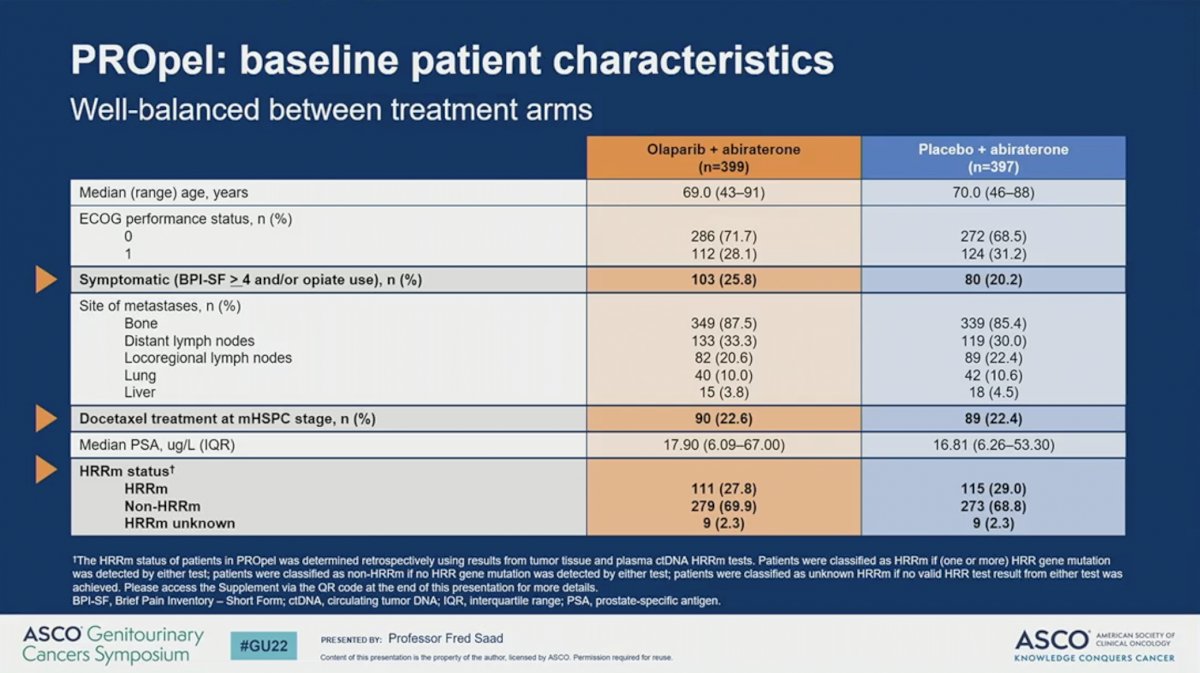
The analytical cohort included 399 patients randomized to receive abiraterone plus olaparib and 397 randomized to receive abiraterone plus placebo. The two treatment arms were well-balanced for baseline patient characteristics. Approximately 23% of patients on both arms received docetaxel for mHSPC. A slightly higher proportion of patients on the olaparib arm had symptomatic disease (26% versus 20%). Similar percentages of patients on the olaparib arm (28%) and placebo arm (29%) had HRR mutations and only 2.3% of patients on each arm had unknown HRR mutations status. Of note, HRR mutation status was defined through tissue or cell-free DNA profiling.
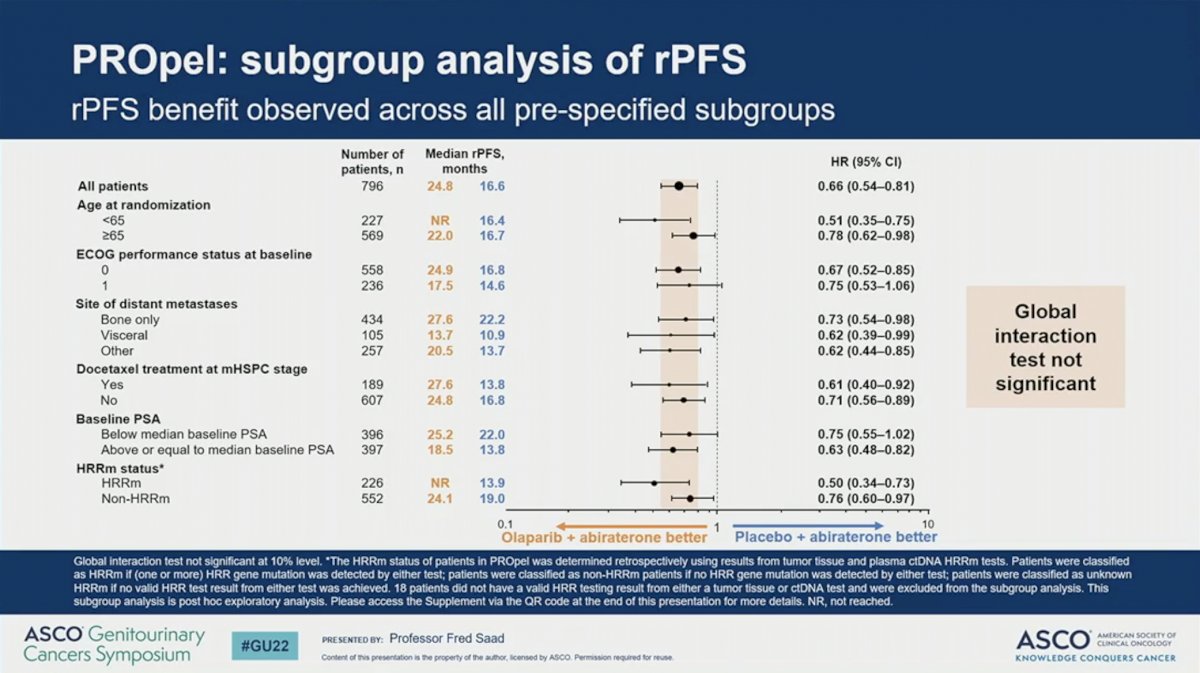
PROpel met its primary endpoint with the addition of olaparib to abiraterone resulting in a 34% reduction in progression or death in (rPFS HR = 0.66, 95% CI 0.54-0.81; P < 0.0001). The addition of olaparib improved median rPFS by 8.2 months (24.8 versus 16.6). This finding was confirmed on blinded independent central review, on which olaparib resulted in a 39% improvement in rPFS and 11.2 month improvement in rPFS.


The observed rPFS was statistically consistent across all pre-specified subgroups. Global interaction tests were not significant for any of the subgroups. Notably, however, the HR was 0.50 (95% CI 0.34-0.73) for patients with HRR mutations and 0.76 (95% CI 0.60-0.97) for those without HRR mutations, suggesting that patients with HRR mutations may derive greater benefit.
The OS data is immature. Only 28.6% of the necessary events have occurred. The preliminary data suggests a trend towards improved OS with a HR of 0.86 (95% CI 0.66-1.12; P = 0.29) with the addition of olaparib to abiraterone.

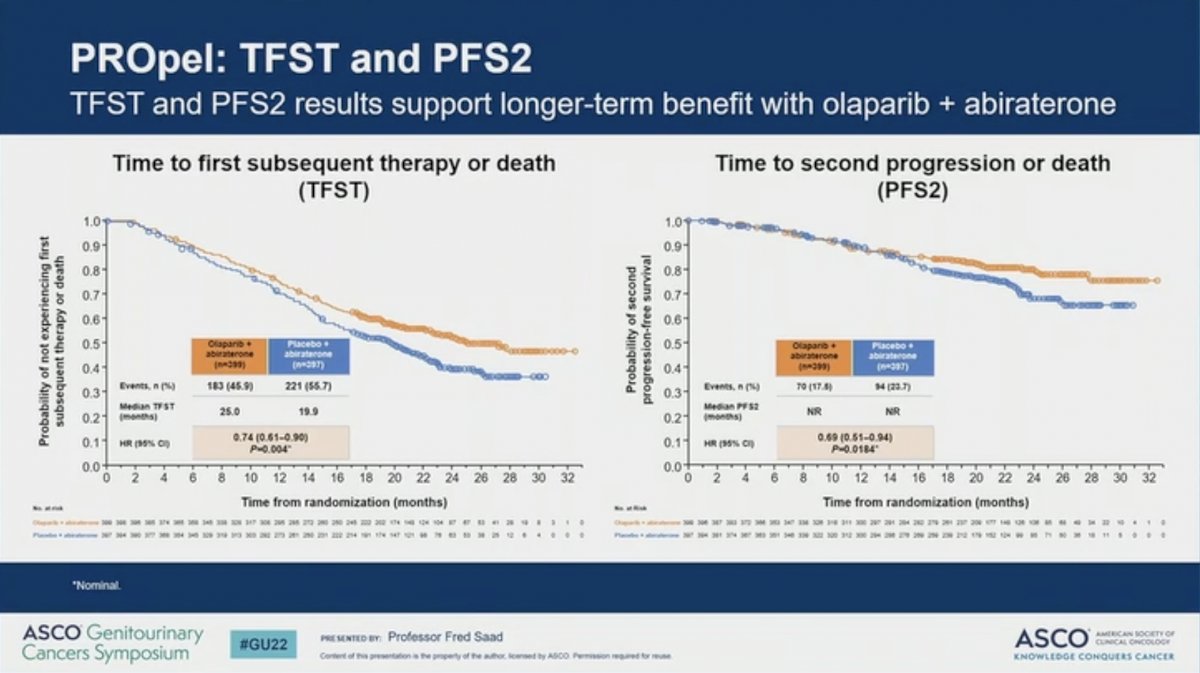
Other secondary endpoints, including time to first subsequent therapy and time to second progression also favored the addition of olaparib to abiraterone.
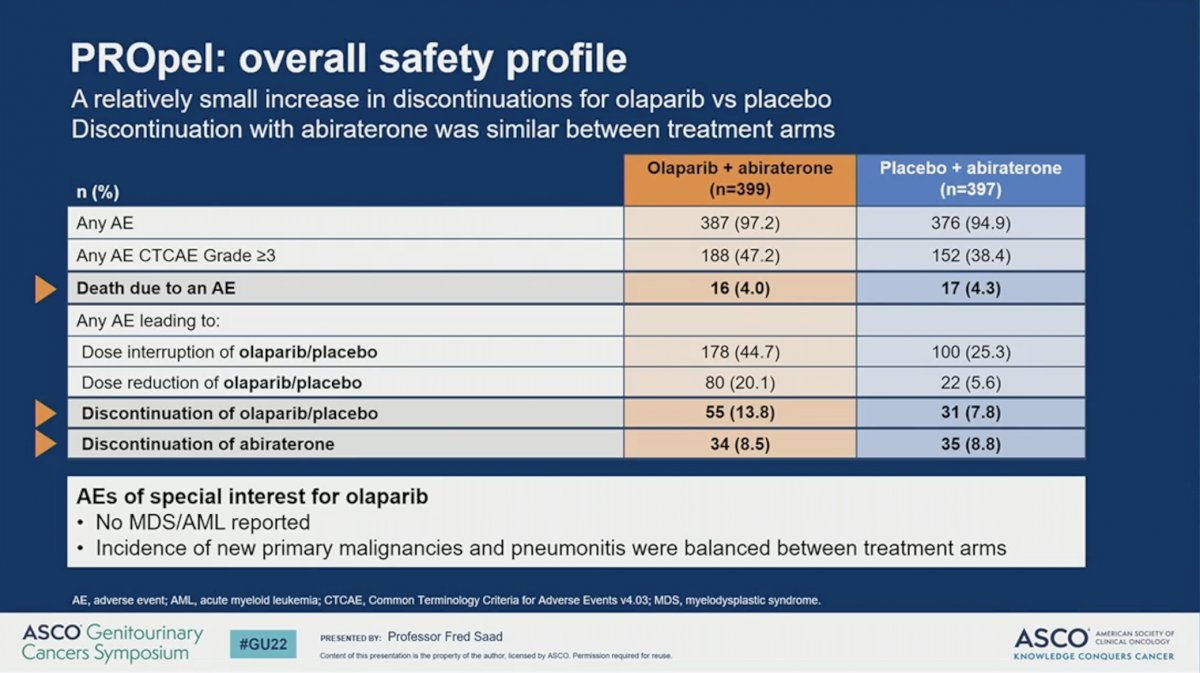
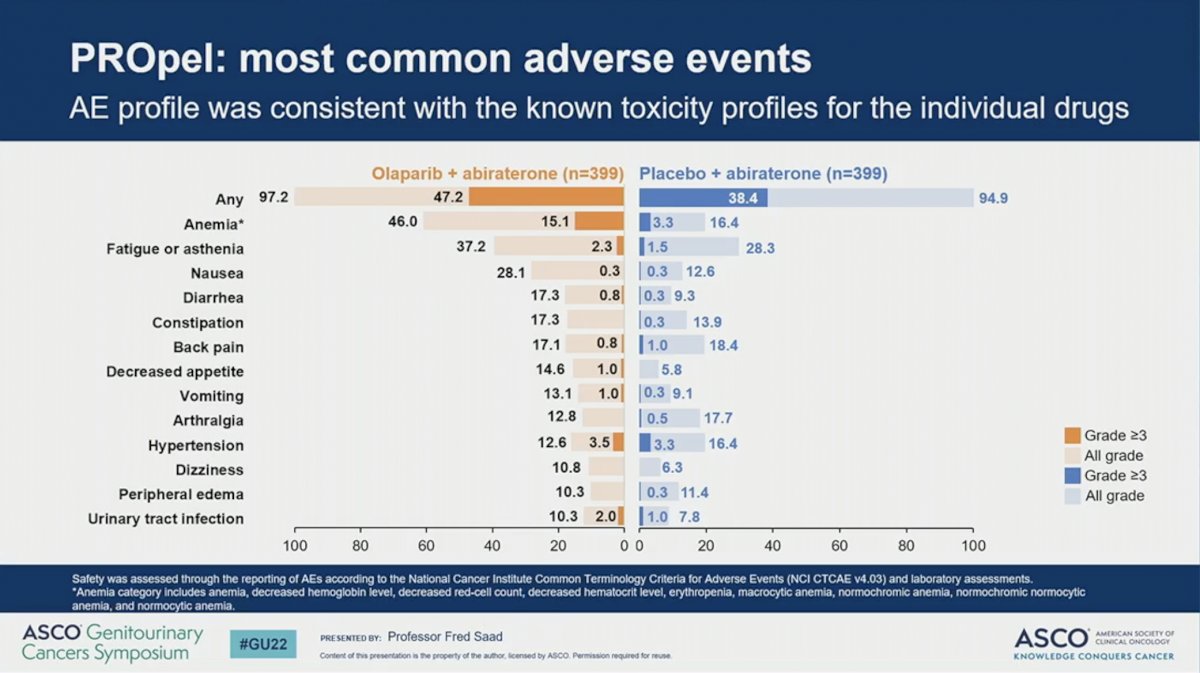
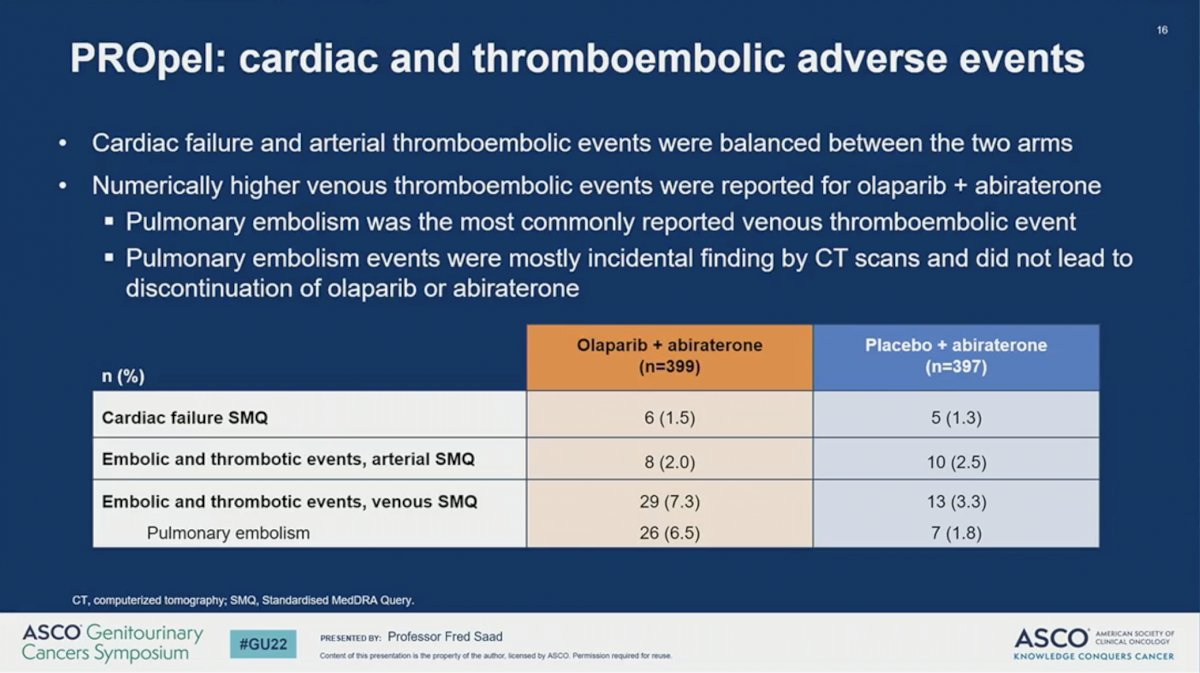
The combination of abiraterone plus olaparib resulted in similar toxicity as previously reported. The percent of patients with any adverse event (AE) or death due to AE were similar across the two arms. A higher proportion of patients receiving abiraterone plus olaparib (47%) had grade 3 or higher AE compared to those receiving abiraterone plus placebo (38%). While a higher percent of patients discontinued olaparib than placebo (13.8% versus 7.8%) there was no difference in the rate of discontinuing abiraterone between the two arms (8.5% versus 8.8%). No incidence of MDS or AML were reported and the incidence of new primary malignancy and pneumonitis were balanced between the two arms. The most common AE observed in patients receiving olaparib was anemia, which occurred in 46% of patients and was grade 3 or higher in 15%. A numerically higher percent of patients treated with abiraterone plus olaparib developed venous thromboembolism, but these tended to be incidentally found and did not result in a higher rate of treatment discontinuation. Quality of life, as measured by the FACT-P scale, remained comparable between the two arms during the study period.
Dr. Saad concluded by summarizing that abiraterone plus olaparib led to a significantly and clinically meaningful improvement in rPFS over abiraterone plus placebo in the first-line mCRPC treatment setting and that the benefit was irrespective of HRR mutation status. The safety profile of abiraterone plus olaparib was consistent with the safety profile of the individual drugs and there was no detriment to quality of life allowing most patients to stay on therapy. Future presentations showing the mature OS data and more granular analysis of the HRR mutant and non-mutant subgroups will be important to determine which patients will benefit most from this combination treatment strategy.
Written by: Fred Saad, MD, FRCS, Centre Hospitalier de l’Université de Montréal/CRCHUM, Montreal, Quebec, Canada
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist at the Dana-Farber Cancer Institute (Twitter: @jberchuck), during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday, Feb 17 – Saturday, Feb 19, 2022
References:
- Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Roubaud G, Özgüroğlu M, Kang J, Burgents J, Gresty C, Corcoran C, Adelman CA, de Bono J; PROfound Trial Investigators. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.
- Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Fléchon A, Redfern C, Goessl C, Burgents J, Kozarski R, Hodgson D, Learoyd M, Saad F. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018 Jul;19(7):975-986.


