(UroToday.com) Best management of oligometastatic disease remains an open question, namely two approaches: metastasis-directed therapy (MDT) in the absence of systemic therapy and systemic therapy alone. The authors note multiple phase III studies demonstrating benefit in both progression-free and overall survival (PFS, OS) to enhanced hormone therapy, yet phase II studies supporting the use of MDT for extending PFS. The selection of patients with – and definition of – oligometastatic disease is changing as sensitive PSMA-based PET scans have become more common place.
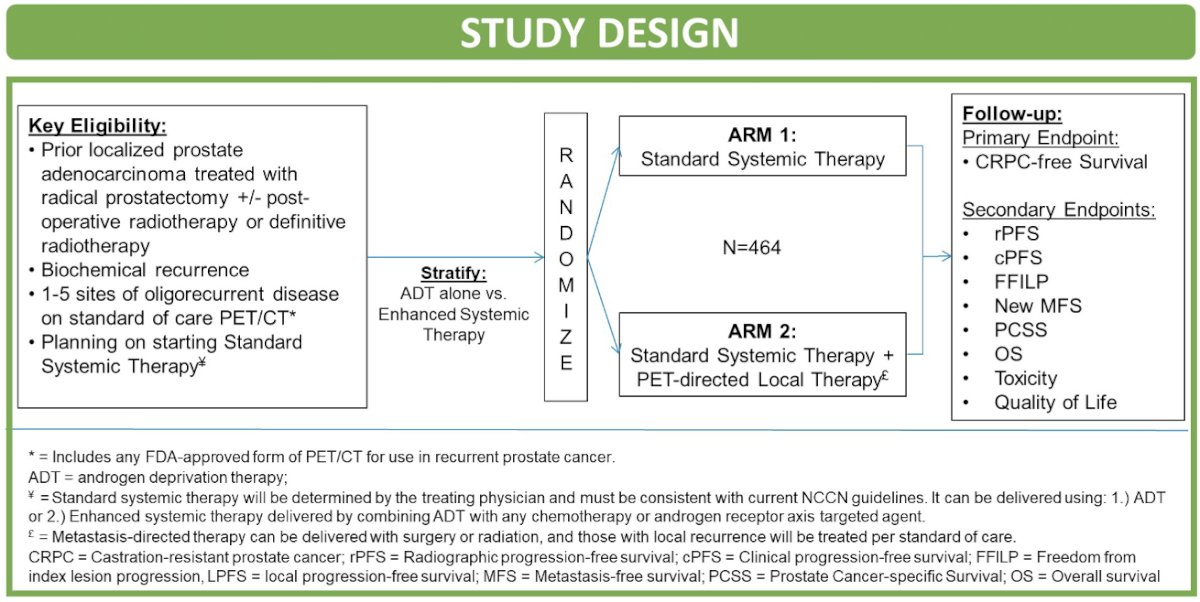
The authors developed the VA STARPORT study (VA Seamless Phase I/II randomaized trial of STAndard Systemic theRapy with or without PET-directed Local thearpy for OligoRecurrenT Prostate Cancer) which has the primary goal of determining if adding PET-directed local threapy to standard systemic therapy improves disease control compared to SST alone in Veterans with oligorecurrent prostate cancer.
The format of the study is a phase II/II randomized trial open at 16 Veterans Affairs (VA) medical centers. All patients will have had biochemical recurrence (BCR) following curative-intent local therapy. As a part of standard work up for BCR, patients will have 1-5 metastatic lesions via an FDA-approved PSMA PET-CT (defined as oligorecurrence). Patients in Arm 1 will receive standard system therapy only. Patients in Arm 2 will also receive surgery or radiation to metastasis and any prostate/prostatectomy bed local recurrence, as well as standard systemic therapy. Metastasis-directed radiation can consist of stereotactic body radiotherapy (SBRT) or elective nodal radiotherapy per clinician discretion in accordance with protocol-permitted dose/fractionation options. The standard systemic therapy is defined as any intended indefinite systemic therapy concordant with NCCN guidelines.
Presented by: Abhishek A. Solanki MD, MS, Hines VA, Hines, IL
Written by: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday, Feb 17 – Saturday, Feb 19, 2022


