(UroToday.com) The unmet need for additional options of therapies in metastatic castration resistant prostate cancer (mCRPC) is readily apparent to every patient and oncologist alike. Continued drug development to find safe and effective therapies is critical for future patient care. Dr. Markowski provides results of a phase Ib/II study of a novel oral agent called sabizabulin, also known as VERU-111.1 Sabizabulin has two purported mechanisms of activity. The first is in crosslinking of the cytoskeleton to inhibit microtubule assembly, which can be androgen receptor (AR) dependent. The second is via disruption of AR translocation along cytoskeletal elements.

The phase Ib portion of the study included 39 patients in a 3+3 dose escalation schema with further expansion of both dose and duration.1 Dosing ranged from 4.5 mg to 81 mg for 7 days as part of a 21 day cycle, followed by continues dosing. The recommended phase II dose (RP2D) was 63 mg PO daily. The phase II portion enrolled 41 patients at this dose. Of note the phase Ib included patients with progression on at least one oral AR targeting agent irrespective of prior receipt of one line of taxane chemotherapy. In the phase II, no prior taxane therapy was permitted.

Here the authors present the data from the 80 patients treated on the combined phase Ib/II study, evaluating best clinical response (BCR) as either an objective response (via PCWG3 critiera) and/or stable disease defined as at least 5 cycles (≥15 weeks) of continuous treatment. BCR was observed in 37.5% (30/80) patients with 5 patients remaining on study for greater than 30 months. Of those patients with measurable disease at treatment initiation, BCR was 59% (17/29). Of these 30 patients, 11 (37%) had received and progressed on at least two AR targeting agents.
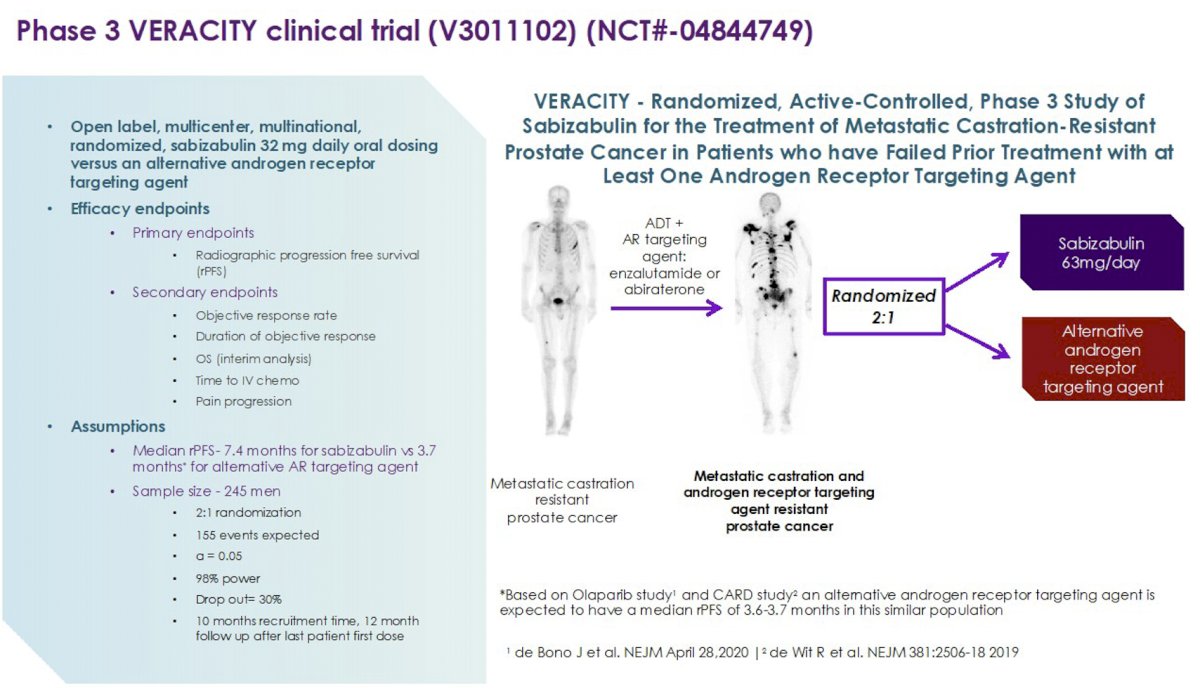
These data suggest that following progression on AR targeting agents – including greater than one – patients can respond to cytotoxic chemotherapy delivered by mouth and targeting the cytoskeleton. Previous data was reported on safety profile, which the authors highlight to not include clinically relevant neutropenia or neurotoxicity. The open and recruiting randomized phase III VERACITY (Efficacy Evaluation of VERU-111 for mCRPC in Patients Who Have Failed at Least One Androgen Receptor Targeting Agent) will compare efficacy of sabizabulin to second ARTA following progression on ARTA.2
Presented By: Mark Christopher Markowski, MD Ph.D., The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD
Written By: Jones Nauseef, MD, PhD, Assistant Professor of Medicine within the Division of Hematology and Medical Oncology, Sandra and Edward Meyer Cancer Center, and Englander Institute for Precision Medicine Weill Cornell Medicine and Assistant Attending physician at NewYork-Presbyterian Hospital. @DrJonesNauseef on Twitter during the 2022 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 17 – Sat, Feb 19, 2022.
References:2. VERACITY - Randomized, Active-Controlled, Phase 3 Study of VERU-111 for the Treatment of Metastatic Castration-Resistant Prostate Cancer in Patients Who Have Failed Prior Treatment With at Least One Androgen Receptor Targeting Agent


