(UroToday.com) In the Rapid Abstract Session on the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Dr. Fizazi presented a rapid abstract of the initial results of the phase I CYPIDES trial, describing the first-in-human results using ODM-208 in men with castration-resistant prostate cancer (mCRPC).
ODM-208 is a novel, oral, non-steroidal, and selective inhibitor of CYP11A1, the first and rate-limiting enzyme of steroid biosynthesis. Through inhibition of CYP11A1, ODM-208 suppresses the production of all steroid hormones and their precursors that may activate the androgen receptor (AR) signaling pathway.
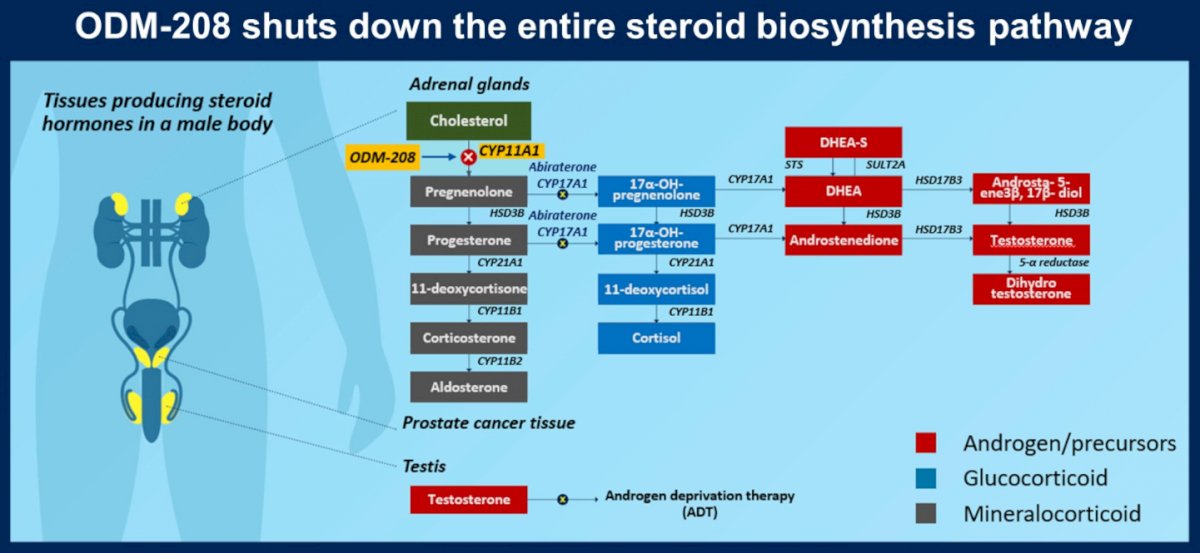
This mechanism of action is particularly relevant for patients with activating somatic point mutations in the AR ligand-binding domain (LBD). Such mutations are a known mechanism of resistance to hormone-based therapies in mCRPC.
The CYPIDES trial utilized a phase I dose-finding 3+3 design among men with progressive mCRPC who have previously received ≥1 line of AR signaling inhibitor and ≥1 line of taxane-based chemotherapy. Patients received ODM-208 up to 150 mg/day with glucocorticoid and mineralocorticoid replacement therapy and androgen deprivation therapy (ADT).

In this phase 1 design, the endpoints of interest were dose-limiting toxicities (DLTs), adverse events, pharmacokinetics, pharmacodynamics, PSA and RECIST response, and exploratory genetic profiling.
As of the data cut-off, 44 patients were treated with ODM-208. The median age of included patients was 70 years. Among the 44 patients, 24 (55%) patients had previously received both abiraterone and enzalutamide, and all had received at least one taxane-based chemotherapy regime.
With the accrual of 41 patients, dose-finding was completed with doses ranging from 10 to 150 mg/day. ODM-208 plasma exposure was dose-proportional.
In terms of biochemical correlates of treatment, serum testosterone was undetectable after 4 weeks of start of ODM-208 in nearly all patients, as were other metabolites including serum DHEA sulfate, androstenedione, 11β-hydroxyandrostenedione, 11-ketotestosterone, and pregnenolone.

The authors hypothesized, as alluded to above, that ODM-20 may be more effective in men with AR mutations as it blocks the production of all AR ligands. This was validated as ODM-20 produced robust PSA responses, particularly among men with mutations in the AR ligand-binding domain.
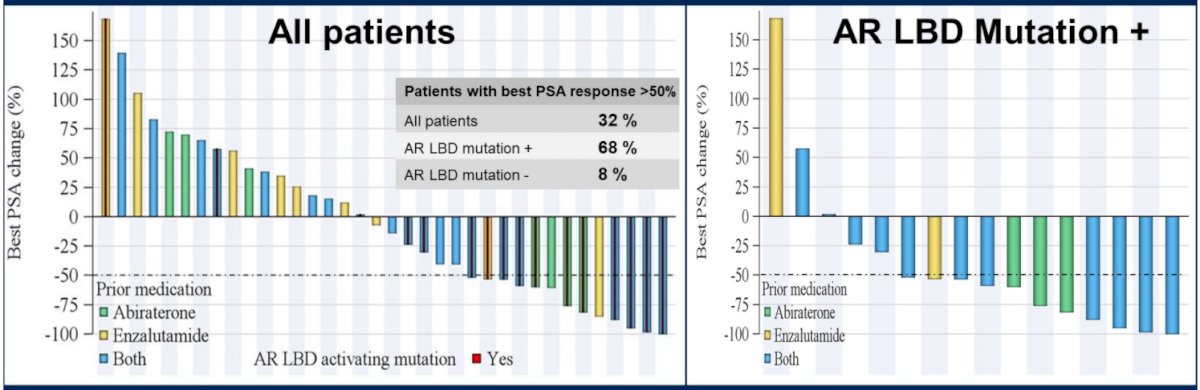
In terms of preliminary oncologic efficacy, 32% of evaluable patients achieved a PSA decline of ≥50%. This rate was higher (68%) in patients with activating somatic point mutations in the AR ligand-binding domain than in those men without (8%).
Although tolerated by most patients, the main safety finding was adrenal insufficiency with grade 3 adrenal insufficiency noted in 14/44 (32%) patients. These patients required short-term high-dose glucocorticoid treatment and required hospitalization. A single dose-limiting toxicity occurred at the 50mg BID dosage. Three patients died during the study, all attributable to prostate cancer.
Dr. Fizazi concluded that treatment with ODM-208 among men with heavily pre-treated mCRPC was highly effective in blocking the production of steroid hormones and showed promising antitumor activity. Based on this phase I results, the phase 2 dose-expansion portion of the CYPIDES is ongoing (NCT03436485).
Presented By: Karim Fizazi, PhD, MD, Gustave Roussy, and University of Paris-Saclay

