(UroToday.com) In the Rapid Abstract Session on the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022, Dr. Gao presented results of a phase 1/2 study examining the role of ARV-110 in metastatic castration-resistant prostate cancer (mCRPC).
While there are numerous agents which have demonstrated improvements in overall survival for patients with mCRPC, decreasing androgen receptor dependence of tumors upon successive therapies limit treatment options and treatment response. ARV-110 is a first-in-class, oral PROteolysis TArgeting Chimera (PROTAC) protein degrader that selectively targets the androgen receptor and, in phase 1 trials, has demonstrated clinical activity in patients with mCRPC who have been heavily pre-treated.
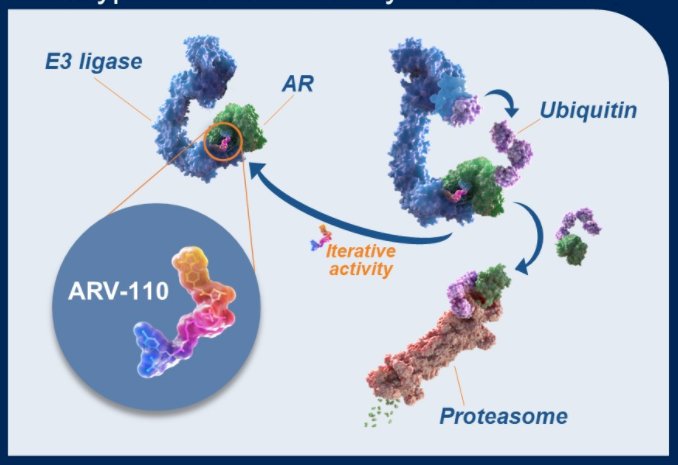
These studies have further suggested that patients with specific molecular profiles, eg, AR T878 and H875 mutations, have an enhanced response to ARV-110. On the basis of these data, an ongoing phase 2 expansion presented here seeks to define the response of ARV-110 in biomarker defined patient subgroups.
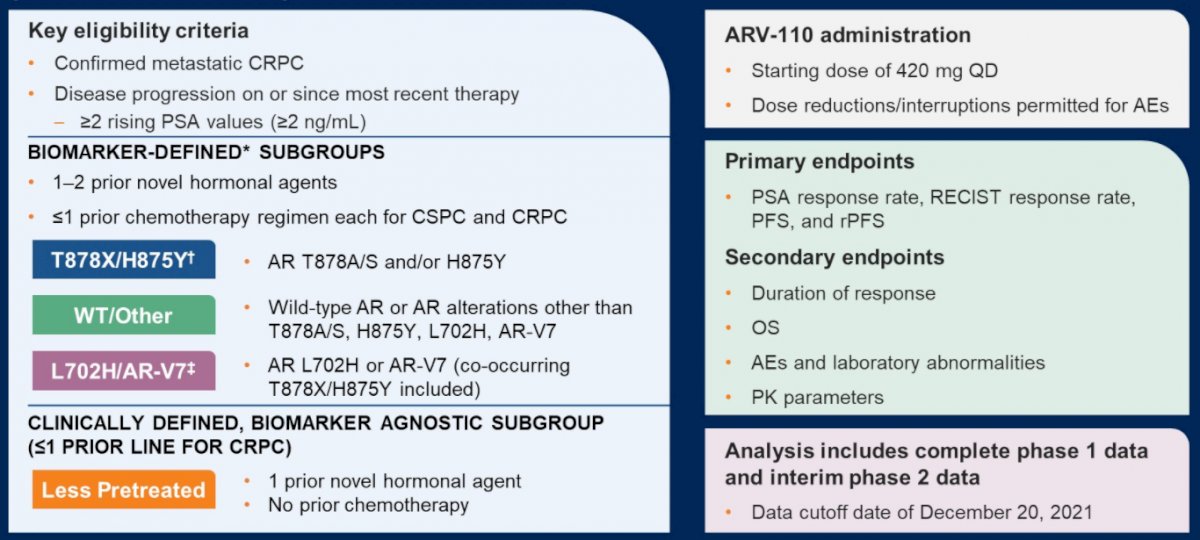
During the phase 1 portion of this study, patients with mCRPC who had evidence of disease progression following two or more lines of therapy (including at least enzalutamide or abiraterone acetate) received ARV-110 orally once or twice daily (QD or BID) in sequential cohorts (3 + 3 dose escalation design). In this phase, the primary objectives were to assess ARV-110 safety and select the recommended phase 2 dose (RP2D).
In phase 2, patients with mCRPC who had received 1 or 2 prior novel hormonal agents with or without chemotherapy were assigned on the basis of 3 biomarker-defined subgroups: 1) AR T878 and/or H875 mutations, 2) AR L702H mutation or AR-V7 (variants not degraded by ARV-110 in nonclinical studies), and 3) wild-type AR or other AR alterations. An additional fourth subgroup enrolled patients with less prior therapy – one of fewer lines of therapy for mCRPC, including one prior novel hormonal agents and no chemotherapy. In this phase 2 study, the primary objective was to assess ARV-110 antitumor activity.
As of a data cut-off of December 20, 2021, 195 patients had been enrolled, including 71 in phase 1 and 124 in phase 2. All patients had received at least one novel hormonal agent. In phase 1, 75% of patients had also received chemotherapy whereas only 31% had in phase 2.
In the phase 1 portion of the trial, ARV-110 doses ranged from 35–700 mg QD or 210–420 mg BID. Based on combined safety, pharmacokinetics, and efficacy data, a dose of 420 mg QD was selected as the RP2D.
There were no grade ≥4 treatment-related adverse events (TRAEs) in 138 patients treated at the RP2D. The most common any grade TRAEs at the RP2D were nausea (48%; grade 3: 1%), fatigue (36%; grade 3: 1%), vomiting (26%; grade 3: 1%), decreased appetite (25%; grade 3: 0), diarrhea (20%; grade 3: 2%), and alopecia (14%). Treatment was discontinued as a result of adverse events in 12 patients (9%).
Across biomarker-evaluable patients in phase 1 and phase 2 who had at least 1 month of prostate-specific antigen (PSA) follow-up, 46% of patients with AR T878A/S and/or H875Y mutations had best PSA declines ≥50% (PSA50).
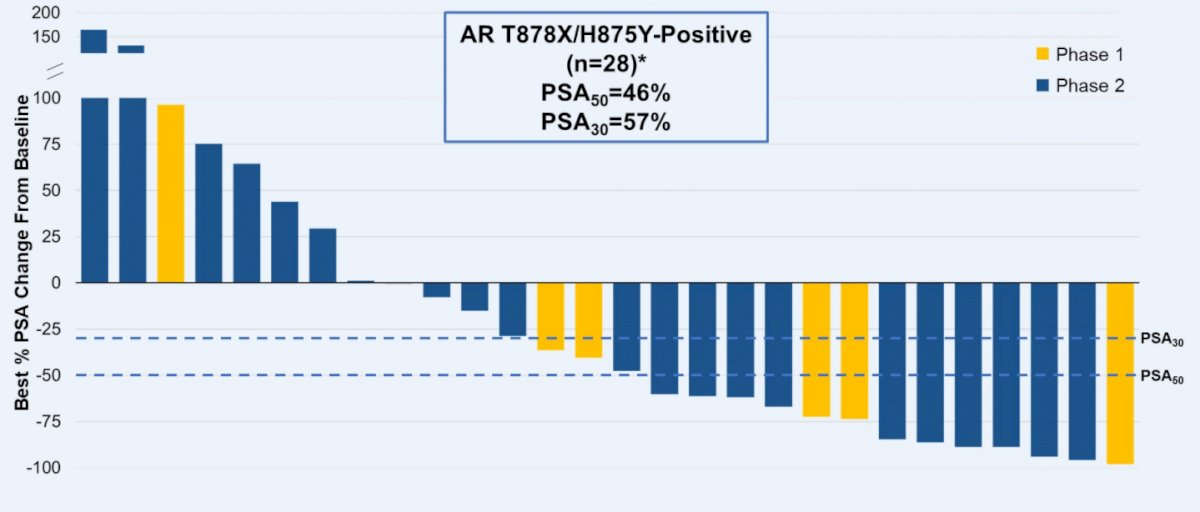
Further, of 7 patients with AR T878A/S and/or H875Y mutations who were RECIST-evaluable, 6 had tumor shrinkage (2 with confirmed partial responses), and 9 of 28 remain on treatment. Notably, 12 patients received treatment for 24 weeks or longer.
Additionally, PSA declines of 50% or greater were seen across all biomarker-defined subgroups including wild-type and less pre-treated patients.
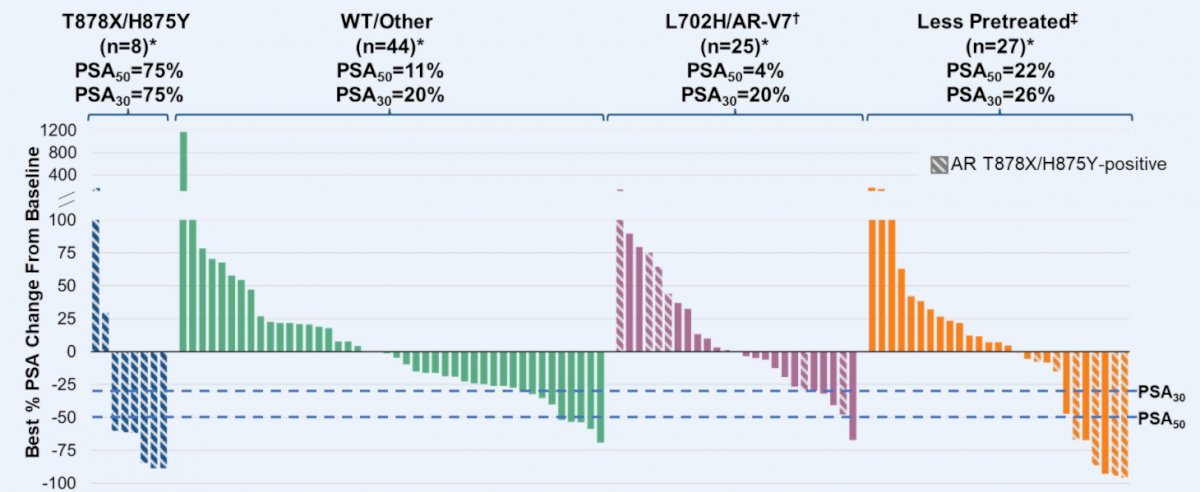
Dr. Gao concluded that the novel AR protein degrades ARV-110 demonstrates clinical activity in a post-NHA, heavily pretreated mCRPC patient population. Additionally, biomarker selection of patients with AR T878 and/or H875 mutations enriches ARV-110 sensitivity.
Presented by: Xin Gao, MD, Massachusetts General Hospital


